

Summary
Background: Therapeutic pneumoperitoneum was first described for overcoming pulmonary tuberculosis in the end of 19th century. However, in time, another indication “prolonged air leak” (PAL) after major lung resections, lung volume reduction surgery and decortication operations have come on the scene and this legendary treatment have took place again. Despite the developing technology, medical devices and tools thoracic surgeons still sometimes need to use this treatment to cope with PAL.Materials and Methods: Twelve patients underwent pulmonary operations were included. Nine of the patients had PAL and three had massive air leak with subcutaneous emphysema in early postoperative period. On mean postoperative 8th day (1-15), a one session pneumoperitoneum was applied. In order to fulfill the vascular bed to prevent from air embolism, all of the patients received 500-1500cc of intravenous saline so that the venous pressure is between 7 and 12.
Results: All 12 patients developed no sequel or chronic complications due to the intervention. Mean hospital stay after the intervention was 8.1 days (2-12), mean chest tube removal time after pneumoperitoneum was 16 days (2-48). Six of the patients were discharged with a complete success (without a chest tube), five patients were discharged with a Heimlich valve and one with passive drainage catheter, but the latter patients were also fully recovered in following days.
Conclusions: In this report, we present 12 cases treated successfully with therapeutic pneumoperitoneum without any severe complications. The unique side of our technique is we used only single application without any need of extra instruments.
Introduction
Introducing air into peritoneal cavity to treat a lung disease is a very old procedure, which was first used to treat tuberculous peritonitis in 1890’s. But it has begun to be used in the treatment of pulmonary tuberculosis in 1930’s [1]. Since the early 1990’s, the idea of the use of therapeutic pneumoperitoneum (PP) for the treatment of PAL after lung resections had come on the scene.One of the most frequent complications after lung resection is PAL. This complication is reported to be seen up to 50% after lung volume reduction surgery [2]. PAL causes longer hospital stay and if present, required adjuvant treatment is delayed because of the undrawn chest tube [3].
In English literature, there are many reports, which describe perioperative and postoperative application of pneumoperitoneum, to cope with present or probable pleural air space problems. While some authors suggest repeated application, our study shows that a single postoperative application with proper technique and timing is enough for a successful treatment [3-8].
There are reports both suggesting intraoperative or postoperative application of therapeutic pneumoperitoneum. In addition, many reports suggest repeated pneumoperitoneum sessions by defined intervals. On the other hand, therapeutic pneumoperitoneum have been described for the residual air spaces or PAL after anatomic lung resections, lung volume reduction surgery and total decortication operations. In this study, we aimed to show the success of just one postoperative application of pneumoperitoneum. What makes our study unique is that it includes different types of resections such as upper lobectomy, lower lobectomy, bilobectomy inferior and also decortication operations performed with either thoracotomy or VATS [9].
Methods
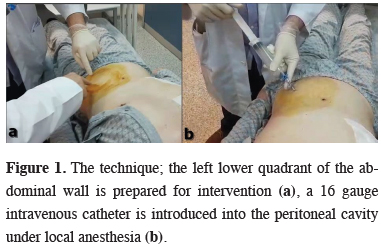
Between January 2015 and August 2018, twelve patients (ten males and two females) underwent pulmonary operations in Ankara Atatürk Chest Diseases and Thoracic Surgery Training and Research Hospital were included in this study. The study protocol was approved by the local Institutional Review Board (642/2019), informed consent was obtained from each patient before the inclusion in the study.Continuous air leak on postoperative day 5 or further was evaluated as postoperative PAL. Nine patients had PAL and three had massive air leak with subcutaneous emphysema in early postoperative period. Bubble exertion through underwater seal drainage bottle even in rest, without any effort, was defined as “massive air leak”. The mean age was 56.8 (18-74). Ten of the patients were operated via thoracotomy while two patients underwent video-assisted thoracic surgery (VATS). There were three lower lobectomy (one right and two left sided), four upper lobectomy (all right sided), two lung volume reduction surgery (LVRS, right and left one each), two total decortication and one bilobectomy inferior operations performed. On mean postoperative 8th day (1-15), under local anesthesia, 1000-1500 cc of room air was introduced percutaneously to left lower quadrant of the abdominal wall, into the peritoneal cavity via a 16 gauge intravenous catheter (Figure 1).
 Click Here to Zoom |
Figure 1: The technique; the left lower quadrant of the abdominal wall is prepared for intervention (a), a 16 gauge intravenous catheter is introduced into the peritoneal cavity under local anesthesia (b). |
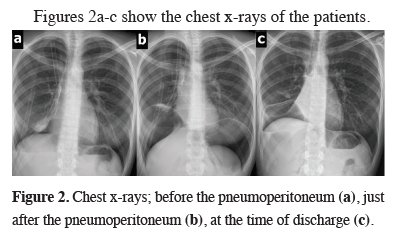
Figures 2a-c show the chest x-rays of the patients.
 Click Here to Zoom |
Figure 2: Chest x-rays; before the pneumoperitoneum (a), just after the pneumoperitoneum (b), at the time of discharge (c). |
The amount of introduced air was decided by the pain development on the shoulder, which shows the phrenic nerve irritation. The night before the intervention, peripheral venous pressure (PVP) was measured. In order to fulfill the vascular bed to prevent from air embolism, all of the patients received 500-1500 cc of intravenous saline so that the venous pressure is between 7 and 12. Chemical pleurodesis was added to treatment in four patients. While four of the patients had malignant disease the remaining were operated for benign conditions.
Results
Clinical characteristics of the patients are shown in table 1.Table 1: Patient characteristics, side and type of the resection, histopathologic diagnosis.
One of the patients had a syncope attack on the time of administration, but he rapidly improved clinically without any further intervention. Thus there was no signs of an air embolism we thought it was a vasovagal syncope. There were no acute complications for the remaining eleven patients. All 12 patients developed no sequel or chronic complications due to the intervention. Mean hospital stay after the intervention was 8.1 days (2-12) and mean chest tube removal time after pneumoperitoneum was 16 days (Table 2).
Table 2: The characteristics of the pneumoperitoneum (PP) intervention.
While six of the patients were discharged with a complete success and without a chest tube, five patients were discharged with a Heimlich valve an one with passive drainage catheter, but the latter patients were also fully recovered in following days.
Discussion
Air leak after pulmonary resection is a widely seen (8-26%) postoperative complication, which causes longer hospital stay and delays adjuvant treatment of oncologic cases. Undoubtedly, the emphysema and the presence of a chronic obstructive lung disease (COPD) are the leading risk factors for PAL after surgery, there are some other risk factors such age, infections, associated interstitial disease, diabetes mellitus, received neoadjuvant induction therapy and malnutrition. Also the intraoperative anatomic conditions such as incomplete fissures and the type of resection (lung volume reduction surgery, upper lobectomy or bilobectomy) is effective in the development of postoperative pleural space problems [6].Some of the treatment strategies to cope with the residual pleural space or the PAL include pleural drainage with or without suction, pleurodesis, endobronchial one-way valves, pneumoperitoneum and redo surgery [6,7].
Historically pneumoperitoneum as a treatment method of pulmonary tuberculosis was used first in 1930’s [1]. Anderson has explained the physiologic aspects of pneumoperitoneum in 1948. An increased intraperitoneal pressure directly transmits to the intrapleural space because of the existing negative intrapleural pressure, thus after only one injection of intraperitoneal air, diaphragm will rise nearly two centimeters [10]. Also Kory and Harden were discussed the physiological effects of pneumoperitoneum widely and they showed that there are no significant negative effects of this treatment on pulmonary and cardiac functions [11,12]. Sure, those days, the most frightening complication of the therapeutic pneumoperitoneum was air embolism. In the report by Atwell et al. it is mentioned that 74 of the 127 cases reported in the literature have been fatal [1]. There are 2 types of air embolism; the pulmonary or venous type and the cerebral or arterial type [13]. In the first type, the site of entry of the air bubble is a systemic vein while in the second type the site of entry is a pulmonary venous channel [14]. A large volume of air is necessary to be fatal in the first type but just a little amount of air may be fatal in the second arterial type. So, one must be careful about preventing to puncture a vascular structure during the intervention. Amar and colleagues have declared that PVP showed a consistent and high degree of agreement with CVP in the perioperative period in patients without significant cardiac dysfunction [15]. In our study, to prevent air embolism, the night before the application we measured peripheral vascular pressure (PVP) of the patients. In order to fill the vascular bed the patient received 500-1500 cc of intravenous saline so that the venous pressure maintained between 7 and 12.
Since the early 1990s, the idea of the use of therapeutic pneumoperitoneum for the treatment of PAL after lung resections has emerged. In a study published by Yusen et al., this method of treatment was reported to be technically feasible [16]. Therefore, in addition to pleural tents, continuous suction drainage, pleurodesis and one-way endobronchial valves; therapeutic pneumoperitoneum has also taken place in the literature in the treatment of long-term air leaks and air spaces. In some of the previous publications, postoperative applications have been reported and in 2003 Toker et al. reported the efficacy of therapeutic intraoperative pneumoperitoneum in patients undergoing lobectomy or bilobectomy for lung cancer [4]. Pneumoperitoneum has been showed to be effective in both apical and basilar space problems after anatomic lung resections and also effective in PAL due to decortication operations and lung volume reduction surgery. Also, intraoperative, postoperative, single or repeated sessions of pneumoperitoneum were all described with their advantages and disadvantages in the literature [2-8,17]. We recommend a single-session postoperative application with an accurate timing. The unique side of our technique is; it does not require neither intraperitoneal pressure measurement nor persistent or adjustable catheters described previously in the literature. Actually only physiologic indicators guide us about deciding the amount of the air we should introduce. First, we take the patient on a supine position. Then, under local anesthesia, a 16 gauge intravenous catheter is introduced into the peritoneal cavity through the left lower quadrant of the abdominal wall (Figure 2). We take care to prevent a vascular injury, and when we are sure that we passed through the peritoneum, we give room air through the catheter. The ending point is the right shoulder pain. When patient describes right shoulder pain, we immediately stop introducing air. The mean air volume we used was 1100 mL (800-1600). The mean increase in the diaphragm was 25.6 mm (8-60) for the right side and 22.1 mm (8-45) for the left side.
If we describe “complete success” as full lung expansion and no chest drain on the time of discharge, then there was a complete success in seven of the patients (50%). But clinically we can also describe discharge with a drainage catheter with or without an Heimlich valve as a “success”, because the PAL lead longer hospital stay, secondary infections, hypoxia and need for mechanic ventilation support. In this manner we were “successful” in all 12 patients (100%). 5 patients discharged with an Heimlich valve and one patient discharged with a passive drainage catheter, but for all of the patients full lung expansion was achieved on following days (7-35 days after discharge). There were no serious complications related to the technique on the follow up.
In conclusion, pneumoperitoneum as a treatment choice of residual pleural spaces and PAL after thoracic surgery operations has been described previously in the literature. The physiologic logic and its feasibility were also discussed. Although there are different reports offering intraoperative pneumoperitoneum, there is no report about the physiologic effect of the perioperative administration. On the other hand there are many reports suggesting repeated sessions of pneumoperitoneum. However, our study showed that a single-session postoperative intervention with an accurate timing was also successful. What makes our technique unique is that, we make the intervention as simple as possible. We use only physiologic indicators to decide how much air volume should be introduced. Therapeutic pneumoperitoneum seems to be safe, feasible, and highly cost-effective treatment for PAL and air spaces. Further studies with higher patient sizes should be conducted to prove the effectiveness of this legendary treatment.
Reference
1) Atwell RJ, Galanti S. Fatal air embolism in therapeutic pneumoperitoneum. Dis Chest 1956; 30: 340-1.
2) Handy JR Jr, Judson MA, Zellner JL. Pneumoperitoneum to treat air leaks and spaces after a lung volume reduction operation. Ann Thorac Surg 1997; 64: 1803-5.
3) De Giacomo T, Rendina EA, Venuta F, Francioni F, Moretti M, Pugliese F, Coloni GF. Pneumoperitoneum for the management of pleural air space problems associated with major pulmonary resections. Ann Thorac Surg 2001; 72: 1716-9.
4) Toker A, Dilege S, Tanju S, Kiyan A, Kalayci G. Perioperative pneumoperitoneum after lobectomy-bilobectomy operations for lung cancer: a prospective study. Thorac Cardiovasc Surg 2003; 51: 93-6.
5) Puc MM, Podbielski FJ, Conlan AA. A novel technique for creation of adjustable pneumoperitoneum. Ann Thorac Surg 2004; 77: 1469-71.
6) Venuta F, Rendina EA, De Giacomo T, Coloni GF. Postoperative strategies to treat permanent air leaks. Thorac Surg Clin 2010; 20: 391-7.
7) Mueller MR, Marzluf BA. The anticipation and management of air leaks and residual spaces post lung resection. J Thorac Dis 2014; 6: 271-84.
8) Podgaetz E, Berger J, Small J, Garza R, Andrade R. Therapeutic Pneumoperitoneum: Relevant or Obsolete in 2015? Thorac Cardiovasc Surg 2017; 65: 375-381.
9) Fındık G, İncekara F, Demiröz M, Sayılır E, İnan K, Hazer S, Aydoğdu K, Kaya S. First experiences and complications in video-assisted thoracoscopic surgery lobectomy at a thoracic surgery center. Turk J Thorac Cardiovasc Surg 2018; 26: 116-122.
10) Anderson NL. The rationale of therapeutic pneumoperitoneum; physiological and mechanical considerations. Dis Chest 1948; 14: 732-8.
11) Kory RC, Roehm DC, Meneely GR, Goodwin RA Jr. Pulmonary function and circulatory dynamics in artificial pneumoperitoneum. II. Studies on patients with pneumoperitoneum as a therapeutic measure in pulmonary emphysema. Dis Chest 1953; 23: 608-20.
12) Harden KA, Hackney R, Quarles C, Jordan A, Payne HM, Johnson Jb. Some observations on the physiological effects of therapeutic pneumoperitoneum upon pulmonary and cardiac function. J Natl Med Assoc 1956; 48: 20-4.
13) Durant TM, Long J, Oppenheimer MJ. Pulmonary (venous) air embolism. Am Heart J 1947; 33: 269-81.
14) Campagna A. Cerebral air embolism complicating therapeutic pneumoperitoneum. Dis Chest 1954; 26: 349-53.
15) Amar D, Melendez JA, Zhang H, Dobres C, Leung DH, Padilla RE. Correlation of peripheral venous pressure and central venous pressure in surgical patients. J Cardiothorac Vasc Anesth 2001; 15: 40-3.



