2Department of Anesthesiology and Reanimation, Bozyaka Training and Research Hospital, İzmir, Turkey DOI : 10.26663/cts.2020.00021
Summary
Although the information increases about the lung damage and radiological findings of SARS-CoV-2 disease day by day, the development of pneumothorax has been reported as a very rare finding, and our information is limited with a few case reports. Here we present a 54-year-old male and a 71-year-old female patient, who were diagnosed spontaneous pneumothorax while they were treating for SARSCoV- 2 pneumonia. A tube thoracostomy performed to each patient for the treatment of pneumothorax. While one case treated with tube thoracostomy successfully prolonged air leak, developed in other patient. Spontaneous pneumothorax should be kept in mind as a differential diagnosis when a sudden hypoxia finding developed in respiratory parameters of patients with SARS-CoV-2. Additional measures also should be taken for these patients during treatment with tube thoracostomy.Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease ongoing since started in December 2019 in Wuhan and declared as a pandemic on 12 March by WHO [1-4].Since the first cases, patients have been reported with pneumonia symptoms like fever, cough, and dyspnea so detection of lung damage has become more important [2-4].Despite severe lung injury and detailed radiological examinations of lungs, the number of patients with pneumothorax obtained very rare and reported as 2% [2,3]. Our experience with pneumothorax treatment is also limited. Treatment methods for patients with prolonged air leak is an undiscussed topic and concomitant pneumonia may limit the chance of surgical treatment. Air leakage from traditional drainage bottles is another issue because of virus spread. In our study, all of these difficulties were tried to open discussion with two case presentation.
Case Presentation
Case 1A 54-year-old male patient, who was followed up with a diagnosis of acute myeloid leukemia, was obtained cough and fever (38.5-39°C) 6 days after consolidation chemotherapy in the hospital. Dyspnea also added to his complaints, and oxygen saturation regressed to (80%) (without oxygen). In biochemical examinations; white blood cell 0.22x103/μL, hemoglobin 8.8 g/dL, hematocrit 22.3%, platelet 13x103/μL, lymphocyte 0.10x103/μL, INR 1.61, D-dimer 485 ng/mL, CRP: 233.2 mg/dL. In arterial blood gas examination, pH: 7.5, pO2: 56.3 mm/Hg, pCO2:35.2 mm/Hg, HCO3 29.3 mmol/L and saturation was: 86.4% with nasal oxygen. Thorax tomography was typical for SARS-CoV-2 pneumonia, with peripheral localized ground glass opacity in the bilateral lungs, more dominant in the lower lobes (Figure 1a).
 Click Here to Zoom |
Figure 1: Radiological examinations of case 1, (a) peripheral consolidations and ground-glass opacities in bilateral lungs, more pronounced on the right, (b) pneumothorax in the right hemithorax, (c) expansion defect after tube thoracostomy. |
After blood, urine, and sputum examples were taken for cultures, a combined throat and nose swab were taken for the PCR test. In addition to empirical antibiotic therapy, hydroxychloroquine, oseltamivir, and favipiravir treatment also started for the preliminary diagnosis of SARS-CoV-2. PCR result was (+), additionally, acinetobacter reproduction was detected in the sputum culture. Acinetobacter reproduction was not obtained in control sputum cultures so the result was evaluated as contamination. In the third day of antiviral treatment, the patient was taken to the intensive care unit (ICU) and intubated because of progression in hypoxia findings. Total pneumothorax was detected on the control x-ray, and a 32F tube thoracostomy was performed without any complication (Figure
Due to continued air leakage, three different methods were used to prevent virus spread from underwater drainage system to ICU air. Primarily, two interconnected drainage bottles were used for the drainage system. Secondly, 70% concentration of ethyl alcohol was placed in the first bottle, and diluted (10%) sodium hypochlorite (NaOCl) was in the other, finally, an HME (bacterial/viral heat and moisture exchanger) was placed in the air outlet cannula (Figure 2).
Air leakage continued without decreasing and surgical resection was not considered due to pneumonic infiltrations and the patient’s general septic condition. Despite all antiviral, antibiotic, and supportive treatments, the patient’s pneumonic consolidations in chest x-ray did not decrease, additionally, D-dimer levels increased near 12,450 ng/ml and procalcitonin levels 43 ng/mL. Autologous blood-patch pleurodesis was attempted on day 12 but had no effect. During the follow up period, multiple secondary problems developed such as lower gastrointestinal bleeding, acute renal failure, peripheral arterial insufficiency, and on day 13, (after tube thoracostomy) patient died due to multiorgan failure and sepsis.
Case 2
A combined throat and nose swab were taken for the PCR test and the result was (+). Empirical antibiotic therapy, hydroxychloroquine, and enoxaparin treatment started. Favipiravir and tocilizumab were also added to her treatment because of increased CRP and D-dimer values and newly developed dyspnea during the follow-up period (CRP: 168 mg/dL, D-dimer: 679 ng/mL). Despite all the treatments, dyspnea progressed and non-invasive mechanical ventilation support was not enough for solve hypoxia problems so the patient was intubated on day 7. On the third day after intubation, mechanical ventilator pressures were increased and on physical examination, decreased respiratory sounds obtained in the right hemithorax. Right pneumothorax detected on chest x-ray (Figure 3b) and then tube thoracostomy applied (Figure 3c). The air leak stopped early in this case and the thorax drainage was terminated on day 4.
Written informed consent was obtained from the patients for publication of their data.

Click Here to ZoomFigure 2: The combined use of traditional drainage bottles to prevent virus spread. The first bottle is connected to a second bottle with a hose. One bottle contains 70% ethyl alcohol and the other bottle contains 10% concentration NaOCl. A viral/bacterial filter has also been added to the air cannula of the last bottle.
A 71-year-old female patient who does not have additional disease except essential hypertension was admitted with complaints of fever and anorexia for 3 days. In biochemical examinations; white blood cell 4.24x103/μL, hemoglobin 14.4 g/dL, hematocrit 43.0%, platelet 179x103/μL, lymphocyte 1.8x103/μL, INR 1.19, D-dimer 282 ng/mL, CRP: 53.9 mg/dL. In arterial blood gas examination, pH: 7.5, pO2: 80.5 mm/Hg, pCO2:34.4 mm/Hg, HCO3 28.5 mmol/L and saturation was 98.0%. Thorax tomography findings were less severe than case 1, but again typical for SARS-CoV-2 pneumonia. There was peripheral localized ground glass opacity in the bilateral lungs, more dominant in the lower lobes (Figure 3a).
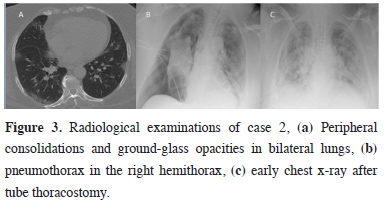
Click Here to ZoomFigure 3: Radiological examinations of case 2, (a) Peripheral consolidations and ground-glass opacities in bilateral lungs, (b) pneumothorax in the right hemithorax, (c) early chest x-ray after tube thoracostomy.
Discussion
Coronavirus pandemic continues to threaten the world and as of May 31, 2020, SARS-CoV-2 has been reported over 5,9 million cases and 367,166 deaths have been confirmed [5]. Although new and different complaints such as anosmia, facial paralysis are defined today, it is known that most patients have lower respiratory tract complaints such as fever, cough, and dyspnea [2-4]. These respiratory complaints have led doctors to use thorax tomography intensively, and today, findings associated with coronavirus pneumonia at the time of diagnosis and follow period have been well identified [3,4].Characteristically known features are bilateral multilobar ground-glass opacification (GGO) with peripheral or posterior dominant distribution and mainly in the lower lobes [3,4,6,7]. Depending on the stage of the disease, septal thickening, bronchiectasis, subpleural involvement, and pulmonary artery thrombosis can be added [3,4,6,7]. Pleural/pericardial effusion, lymphadenopathy, parenchymal cavitation, and pneumothorax has been reported rarely seen in the literature [3]. Chen et al reported 99 patients with pneumonia in their study, and they experienced only 1 case with pneumothorax [2]. Similar, Yang et al examined 52 intensive care patients retrospectively and reported pneumothorax in only one patient [8]. Unfortunately, there is no detailed information about treatment models for pneumothorax in these studies. An interesting case reported by Sun et al in a male patient who did not have a lung disease and had no bulla on thorax tomography before [6]. After SARS-CoV-2 pneumonia diagnosis, a new bulla developed during the follow up period and pneumothorax accompanied. However, in this case, the patient did not undergo tube thoracostomy. Similar to this patient, there was no history of lung disease or a bulla-bleb structure in thorax tomography of our cases too.
Tube thoracostomy experience is also limited in this special patient group and traditional drainage bottle safety is an important topic. As it is known, while using traditional drainage bottles, leaked air passes from alveoli to pleural space than pass from water and reach directly to the atmosphere. During this track, the infected air in the alveoli can pass the intensive care air and this can be a way to spread the virus. With this concern, protective measures were tried to be taken. First of all, the air was passed through the liquid barrier twice by using 2 thorax bottles. At these stages, 70% ethyl alcohol was added to the first bottle and diluted (10%) NaOCl was added to the second bottle. Finally, an HME filter is placed on the air outlet cannula. The minimal resistance of HME to air pass was ignored. During the follow-up of case 1, a negative aspirator was also used for a while to ensure full expansion.
Prolonged air leak is another additional problem in these patients. Aiolfi et al reported successful results with thoracoscopic blep resection in two SARS-CoV-2 and iatrogenic pneumothorax patients for persistent pneumothoraces [9]. In our study, prolonged air leak occurred in only one case and thoracoscopic resection was not considered due to the general septic condition and diffuse lung damage.
Since there was few experience in SARS-CoV-2 in the literature, we could compare our patients with the previous similar coronavirus outbreaks patients for developing another view. In the study reported by Felice about SARS CoV-1 in 2004, the rate of spontaneous pneumothorax was reported as 1.7%. In all patients who applied tube thoracostomy developed prolonged air leakage or recurrence and air leaks took 14 to 31 days (mean of 23.5 days) to resolve [10]. In this respect, prolonged air leak seems predictable because of lung parenchyma inflammation and damage. Severe pneumonic conditions can also hinder the patient’s surgical treatment possibility.
In conclusion, our knowledge about the coexistence of SARS-CoV-2 and pneumothorax is limited. We have a little experience in which patients pneumothorax develops, what have to do in a prolonged air leak and which measures have to be taken to prevent virus distribution from the bottle. Additional measures also should be taken for these patients during treatment with tube thoracostomy. We believe that our study will be a benefit case to start a discussion about these important issues.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Reference
1) World Health Organization (internet). WHO announces COVID-19 outbreak a pandemic. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200531-covid-19-sitrep-132.pdf? Sfvrsn=d9c2eaef_2.
2) Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13.
3) Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus Disease 2019 (COVID-19): A systematic review of imaging findings in 919 patients. Am J Roentgenol 2020:1-7. doi:10.2214/AJR.20.23034.
4) Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W et al. Correlation of chest CT and RT-PCR testing in Coronavirus Disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020:200642. doi:10.1148/radiol.2020200642.
5) World Health Organization (internet). Coronavirus disease 2019 (COVID-19), Situation Report-94. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200423-sitrep-94-covid-19.pdf?sfvrsn=b8304bf0_4.
6) Sun R, Liu H, Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol 2020;21:541-4.
7) Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506.
8) Yang X, Yu Y, Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020 doi: 10.1016/S2213-2600(20)30079-5.
9) Aiolfi A, Biraghi T, Montisci A, Bonitta G, Micheletto G, Donatelli F et al. Management of persıstent pneumothorax with thoracoscopy and blebs resectıon in Covıd-19 patients. Ann Thorac Surg 2020. doi: https:// doi.org/10.1016/j.athoracsur.2020.04.011.
10) Felice GA. SARS, pneumothorax and our response to epidemics. Chest 2004;125:1982-4.






