

2Department of Anesthesiology and Reanimation, Division of İntensive Care, Hitit University, Erol Olcok Training and Research Hospital, Çorum, Turkey DOI : 10.26663/cts.2021.0008
Summary
In patients with acute respiratory distress syndrome (ARDS), mechanical ventilators (MV) are still key devices in the treatment. However, in cases where MV remains insufficient, extracorporeal membrane oxygenation (ECMO) is the most important treatment modality. Venovenous extracorporeal membrane oxygenation (VV-ECMO) was applied to a 42-year-old female patient due to ARDS developed after an in-car traffic accident, and it was successfully terminated. The case was presented to discuss the management of MV and ECMO in patients with ARDS developed after blunt trauma in the light of current literature.Introduction
Mechanical ventilators (MV) constitute the most important part of the treatment in patients with acute respiratory distress syndrome (ARDS) developed after blunt trauma. However, in cases where MV remains insufficient, the use of extracorporeal membrane oxygenation (ECMO) for appropriate patients reduces mortality [1-3].Venovenous ECMO (Vv-ECMO) was applied to a 42-year-old female patient due to ARDS developed after an in-car traffic accident, and it was successfully terminated. The case was presented to discuss when and how MV and ECMO should be performed in patients with ARDS developed after blunt trauma in the light of current literature.
Case Presentation
A 42-year-old woman who was evaluated in the emergency clinic after an in-car traffic accident had a pulse of 85 beats/min, arterial blood pressure of 128/72 mm/Hg, and peripheral oxygen saturation (SpO2) of 95% while breathing ambient air. In her physical examination, she was conscious, breathing sounds were natural. Palpation revealed tenderness in both posterior ribs, left arm, and pelvic area. The biochemical analysis was normal. Complete blood count values were as follows: Hb: 11.5 g/dl, Htc: 35.3%, Wbc: 21700/mm3, Plt: 220/mm3. Arterial blood gas (ABG) values were as follows: pH: 7.39, PaO2: 93 mmHg, PaCO2: 38 mmHg, and HCO3: 24. No hemopneumothorax was observed at anteroposterior chest radiography (APCR) and thoracic computed tomography (CT), whereas there were displaced fractures in the left 3rd-12th ribs and the right 6th and 7th ribs, and a moderate contusion on the left lower lobe posterior (Figure 1).
 Click Here to Zoom |
Figure 1: Anteroposterior chest X-ray and thorax computed tomography showing rib fractures and moderate contusion on the posterior lower lobe on the left. |
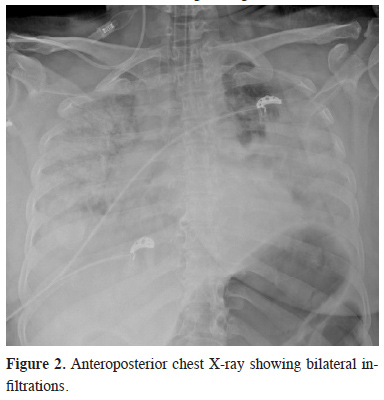
Other X-ray graphs showed fractures in the pelvis and left humerus. On the second day, the thoracic CT showed progression of the pulmonary contusion (SpO2 85%). Despite non-invasive ventilation (NIV), the patient’s clinical condition deteriorated, and the patient was intubated. After observing bilateral infiltrations in APCR (Figure 2), the absence of right heart overload findings on echocardiography (ECHO), the value of PaO2/FiO2 (P/F ratio) below 80, SpO2 not exceeding 45% despite maximum support with MV, and PaO2 not exceeding 40 mmHg in ABG, the patient was diagnosed with ARDS. As a result, Vv-ECMO application was decided.
 Click Here to Zoom |
Figure 2: Anteroposterior chest X-ray showing bilateral infiltrations. |
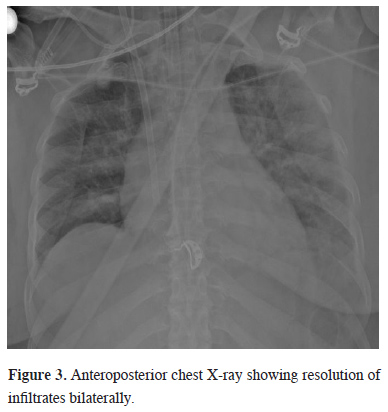
During ECMO noradrenaline infusion was started for the normotensive target. After the first 24 hours, enteral nutrition and an empirical antibiotic therapy were started. Due to resistant hypoxemia on the first day of ECMO, PEEP was set to 14, FiO2 to 100%, pressure support above PEEP to 18 mmHg, and respiratory rate to 16 in P-SIMV mode at MV. The ECMO was set to “full flow” while the heparin infusion was titrated according to the activated clotting time. Upon achieving the target in ABG in the first 24 hours, PEEP remained constant and the FiO2 ratio was gradually lowered first in MV and then in ECMO. ECMO was terminated on the 5th day, when spontaneous respiration was observed and bilateral resolution of infiltrates were seen in APCR (Figure 3). During ECMO, no additional problem was encountered except for the transfusion requirement. However, fiberoptic bronchoscopy was performed on the 3rd day of ECMO due to increased airway pressure in MV and the absence of right lung ventilation.
 Click Here to Zoom |
Figure 3: Anteroposterior chest X-ray showing resolution of infiltrates bilaterally. |
On the 10th day of intubation, the patient who succeeded the spontaneous breathing in trials was extubated. Nasal high-flow O2 and NIV support were given. The patient was provided with respiratory physiotherapy. The patient, on the 3rd day of the extubation, was transferred to the orthopedics clinic on the 21st day of hospitalization for the treatment of her existing fractures.
Written informed consent was obtained from the patient for publication of her data.
Discussion
ARDS is an acute, common, inflammatory lung injury that may be associated with different etiologies. Identifying the patient with ARDS and starting treatment quickly is very important in reducing the high rate of mortality. ARDS should be suspected in patients with progressive dyspnea complaints, increased oxygen demand, and bilateral non-cardiac alveolar infiltration in the lungs within the first 6-72 hours in the imaging. The definition and severity of ARDS were classified in the Berlin Definition [1].No specific treatment has been developed in ARDS. MV support may be necessary for its severe forms. However, ECMO is the most commonly used extracorporeal treatment strategy in severe ARDS cases where MV support is also insufficient [2,3]. ECMO was first successfully used in 1972 in a patient with post-traumatic respiratory failure. According to the records of “Extracorporeal Life Support Organization”, the most important organization related to ECMO, for the last 10 years, the number of cases tends to increase over the years [4,5].
In their review study on the causes and treatments of ARDS in the last 50 years by Yavan et al [6], the researchers revealed that sepsis, aspiration pneumonia, pancreatitis, smoke inhalation, multiple trauma, fat embolism, as well as, blood transfusion, drug toxicity, although less frequently, were the reasons and examined whether they were preventable. In the present case, the presence of trauma causing multiple fractures and a history of blood transfusion may be ranked among the possible causes. In a study conducted by Yuksek et al [7], 72.7% of patients who were subjected to ECMO were observed to be applied to secondary pneumonia. The diagnosis of pulmonary embolism was excluded due to the absence of right overload in echocardiogram, low pulmonary artery pressure, lack of hypotension, and negative Doppler ultrasonography of lower extremity which was performed to exclude the diagnosis. Although thoracic CT angiography was considered for definitive diagnosis, it could not be performed due to hemodynamic instability and ECMO.
The treatment was continued with Vv-ECMO since the patient was young, P/F ratio was below 80 mm Hg, SpO2 not exceeding 45% in MV, and PaO2 not exceeding 40 in MV with maximum support. Although the prone position was recommended to ARDS patients [2,3,5], ECMO was decided to be applied because of insufficient clinical experience for the prone position in our center and the presence of severe and deep hypoxemia. In the guidelines [2,3,5], although intermittent recruitment maneuvers (RM) were recommended in ARDS, RM was kept for a short period due to the presence of hypotension, heparin use during ECMO, an excessive number of rib fractures, and the risk of hemodynamic pneumothorax due to overdistension.
During applying ECMO, it is recommended to provide deep sedation and muscle relaxation to rest the lungs, to prevent air embolism during cannulation, and to reduce oxygen consumption [5]. Neurological examination with sedation interruptions after the first 12-24 hours prevents masking of the complications that may occur. In our case, infusions of muscle relaxant and sedation were given during ECMO. Neurological evaluation was performed by sedation interruptions. Several studies in the literature revealed that neuromuscular blockade reduced the development of MV-associated and MV-induced lung injury (VILI/VALI) by allowing the synchronization of MV and patient and controlling transpulmonary pressures [5]. In a meta-analysis, it was observed that muscle relaxant agents reduced the risk of pneumothorax [8]. In the present case, barotrauma-related pneumothorax was not developed.
The main approach to the use of inotropic and vasodilator agents in ECMO should be to rest the heart by minimizing the need. Low-dose inotrope in a manner that ensures adequate contractility and left ventricular contraction, is recommended [5,8]. In the present case, noradrenaline infusion was given during ECMO to achieve normotensive targets.
In critically ill patients, corticosteroids are used to create a strong anti-inflammatory effect and to prevent corticoadrenal insufficiency [5,8]. In the present case, hydrocortisone was added to the treatment due to post-traumatic ARDS-induced hypotension. Similar to all critically ill patients, a full-calorie and protein dietary support is essential for the patient in ECMO [5,8,9]. Our patient was given enteral nutrition support after the first 24 hours.
Although anticoagulant therapy is recommended to prevent clot formation in the ECMO circuit, heparin-induced bleeding during ECMO is also possible [5-9]. The transfusion is recommended up to the hematocrit level required for adequate oxygen delivery [5-9]. The humerus and pelvic fractures of our case increased the already existing bleeding risk. Although intermittent erythrocyte suspension and fresh frozen plasma replacements were performed, any complication due to heparin infusion was not developed.
In patients who require ECMO, a large part of the lung is collapsed and under consolidation, and only small areas called ‘baby lungs’ remain as functional lung tissue. Since excessive stress and difficulty may be encountered in the lungs due to conventional lung-protective strategies, it is open to the development of VILI [2,3,10]. The most important point in the treatment of ARDS is to monitor low volume and pressure ventilator management while continuing the treatment of the underlying disease [2,3,11]. Pressure controlled modes were also used in ventilator management during ECMO in the present case. Low pressure was maintained during MV by keeping the Ppeak below 30 cmH2O and Pplato below 20 cmH2O. In this way, the driving pressure was kept at the specified levels.
In conclusion, the decision to apply ECMO to the appropriate patients with ARDS and to disconnect the patient from ECMO to eliminate the complications of ECMO should be given before being late. It should be kept in mind that successful ECMO management in a patient with ARDS is multidisciplinary teamwork.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Authors’ contributions
MÖ; Conceived and designed the analysis, co-wrote the paper, collected the data, performed the analysis, DFE; Contributed data/analysis tools, co-wrote the paper.
Reference
1) Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E et al. Acute Respiratory Distress Syndrome. The Berlin Definition. JAMA 2012; 307: 2526-33.
2) Fan E, Del Sorbo L, Ewan CG, Hodgson CL, Munshi L, Walkey AJ et al. An official american thoracic society/european society of ıntensive care medicine/society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2017; 195: 1253-63.
3) Griffiths MJD, McAuley DF, Perkins GD, Barrett N, Blackwood B, Boyle A et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Resp Res 2019; 6: 1-27
4) Morris JA, Pollock R, Zwischenberger BA, Croft B, Zwischenberger JB. The story of ECLS: History and future. In: Schmidt GA editors. Extracorporeal life support for adults. New York: Springer; 2016. p.233-260.
5) elso.org [Internet]. Ann Arbor: Extracorporeal Life Support Extracorporeal Life Support Organization (ELSO) Guidelines for Adult Respiratory Failure, Version 1.4 August; c2017 [cited 2017 Aug] Available from: https://www.elso.org/.
6) Yadav H, Thompson BT, Gajic O. Fifty Years of Research in ARDS. Is Acute Respiratory Distress Syndrome a Preventable Disease? Am J Respir Crit Care Med 2017; 195: 725-36.
7) Yüksel A, Tecimer EM, Özgöz MH, Yolgösteren A, Kan İris İ, Doğan İmran A et al. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: our single-center experience. Turk Gogus Kalp Dama 2017; 25: 61-7.
8) Pappalardo F, Montisci A. Adjunctive therapies during veno-venous extracorporeal membrane oxygenation. J Thorac Dis 2018; 10(Suppl 5): 683-91.
9) Bayar MK, Kosovalı DB. Ekstrakorporeal membran oksijenizasyonu. In: Karadağ M editor. Mekanik Ventilasyonda Son Gelişmeler. Ankara: Akciğer Sağlığı ve Yoğun Bakım Derneği; 2018. p.93-103. Available from: https://ghs.asyod.org/Konular/2018-6-11.pdf.



