Summary
Background: Primary chest wall tumors (PCWT) constitute 5% of all thoracic tumors. The purpose of this study is to determine the frequency, diagnosis and treatment methods of PCWT in a single-center.Materials and Methods: All patients operated between January 2013 and July 2015 in a single center were retrospectively analyzed and patients having chest wall tumors were included in this study.
Results: Among 1073 operations performed during the same period of time, twenty-five patients with primary chest wall tumors were included in this study; 17 were women (68%) and the mean age was 47.6 ± 16.1. The most common symptom was pain (68%). Twenty-four of the lesions were totally excised and reconstructive interventions were added if necessary. Definitive diagnosis was as follows; elastofibroma dorsi in 10 patients (40%), fibrous dysplasia in 3 (12%), and some other rare tumors in the remaining. Twenty-two (88%) of the lesions were benign.
Conclusions: It is observed that the frequency of chest wall tumors is not so high, and most of the lesions are benign which can be resected completely.
Introduction
Chest wall consists of soft tissues, cartilage and bone skeleton. Therefore, chest wall tumors include a wide variety of tumors and more than half of these tumors are reported to be malignant [1]. Most of the chest wall tumors originate from soft tissue [2]. Primary chest wall tumors (PCWT) can be diagnosed with needle aspiration, incisional or excisional biopsy. Wide local excision is recommended to the patients for surgical treatment. Also various reconstruction techniques and materials can be used on patients to stabilize the chest wall and protect the intrathoracic organs [3,4].The purpose of this study is to examine the frequency, diagnosis and treatment methods of PCWT in a single-center study.
Methods
Twenty-five patients having PCWT and operated between January 2013 and July 2015 were analyzed retrospectively. Tumors metastatic to the chest wall and intra thoracic tumors invading the chest wall were not included in the study. The cases were evaluated according to sex, age, localization of the tumor, clinical findings, surgical treatment method, histopathological diagnosis, early and long term results. Posteroanterior chest X-ray, computed tomography (CT), respiratory function test and routine blood tests were requested from the patients before the operation. Bone scintigraphy was obtained for the patients with bone lesion. Operation notes and pathology reports of the patients were reviewed retrospectively.Complete resection was performed for all tumors. Resection with 4 cm. safe surgical margins was achieved in malignant tumors and benign tumors were resected with their capsule. Chest wall defects larger than 5 cm at the anterior chest wall and defects larger than 10 cm at the posterior wall were reconstructed with synthetic material (polypropylene mesh). Patients having benign tumors were followed for 2 years with chest X-rays and thorax computed tomography in every 6 months. Otherwise malignant tumors were followed at least 5 years with computed tomography every 3 months in the first year.
Results
Seventeen (68%) of the patients were woman and the mean age was 47.6 ± 16.1. The most common complaints for approval were pain (68%) and swelling in the thoracic region (44%) (Table 1).Table 1: Preoperative complaints
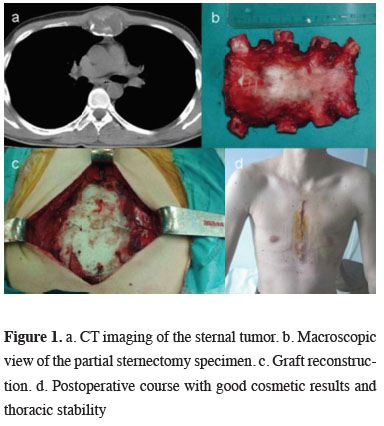
Complete resection was performed in 24 of the patients with PCWT. Incisional biopsy was performed in only one patient. Reconstruction was performed in 2 cases with anterior chest wall abldefects larger than 5 cm. In one of the 2 cases polypropylene mesh was used alone and in the other chest wall was stabilized by sandwich graft technique using methyl methacrylate (Figure 1). In one case bilateral elastofibroma was bilaterally excised in the same session. In one case with elastofibroma the lesion emerging in the opposite side after 2 years was excised. Desmoid tumor recurred after 2 years, it was not operated and radiotherapy was planned.
 Click Here to Zoom |
Figure 1: a. CT imaging of the sternal tumor. b. Macroscopic view of the partial sternectomy specimen. c. Graft reconstruction. d. Postoperative course with good cosmetic results and thoracic stability |
Total excision and reconstructive procedures, according to the intraoperative findings, were performed in 24 of the cases. When postoperative definitive diagnosis was examined, elastofibroma was found in 10 patients (40%), fibrous dysplasia in 3 patients (12%), lipomatous lesions in 2 patients (8%), neurofibroma in 2 patients (8%) and chondroma in 2 patients (8%) (Table 2).
Table 2: Histopathological classification of primary chest wall tumor findings
Twenty-two (88%) of the lesions were benign and 3 (12%) were malignant. None of the patients had received chemotherapy or radiotherapy preoperatively. Recurrence was detected in one patient with desmoid tumor and radiotherapy planned. One patient with plasmacytoma underwent a combined chemotherapy and radiotherapy.
No graft-related complication was observed postoperatively. In the follow-up mortality was not observed.
Discussion
The most common symptoms of chest wall tumors are swelling, pain and palpable mass [1,2,4,5]. The most common cause of PCWT in adults is soft tissue where 68% of the cases originated from [1,2].Chest x-ray, computed tomography and magnetic resonance imaging methods are used for preoperative evaluation. Positron emission tomography is a limited diagnostic method for chest wall tumors but is useful in detecting the most metabolically active area for biopsy and distant metastasis [6].
It is difficult to distinguish clinically and radiologically between benign and malignant chest wall tumors [3]. Therefore, these tumors can be diagnosed with needle aspiration, incisional or excisional biopsy [2,7,8]. We choose to perform excisional biopsy in tumors less than 5 cm and incisional biopsy in tumors larger than 5 cm. In excisional biopsy tumor tissue should not be left on the border of resection.
PCWT constitute 2% of all tumors and 5% of thoracic tumors. The malignancy percentage of PCWT is 60-70%. In our series 12% was malignant and 88% was benign tumors. Benign lesions and originate from soft tissue, and most frequently 40% of the tumors were elastofibroma.
Large defects (anterior defects more than 5 cm, posterior defects more than 10 cm), can be successfully reconstructed after resection. It is stated that postoperative mortality and morbidity were better in the cases with reconstruction [2,9]. Reconstruction was performed in 2 cases with anterior chest wall defects larger than 5 cm. In case with rib resection polypropylene mesh was preferred, and in the other case with partial sternum resection, polypropylene mesh was used and the chest wall was stabilized using the method of sandwich graft with methyl methacrylate.
It is reported that the most frequent locations of solitary plasmacytoma are ribs (62%), clavicle (21%), sternum (12%) and scapula (4%) [5]. Mostly plasmacytoma is seen in the 5th-6th decades as well demarcated lytic lesions like a staple hole [10]. The role of surgery in plasmacytoma cases is only for diagnosis. One of cases was diagnosed via surgical biopsy and the patient had radio and chemotherapy.
Elastofibroma is known as a rare soft tissue tumor of the chest wall [3]. It can be seen in subscapular region, lateral chest wall, deltoid muscle and axilla. It is mostly located in subscapular-infrascapular region, and therefore, it is called as elastofibroma dorsi. It is a slow growing, benign, solid connective tissue tumor with intact capsule. It is a soft tissue mass which is mostly located deep under scapula, makes scapula explicit by pushing it out and whose borders cannot be well-distinguished. Occasionally, it may cause pain in periscapular region, tension and limitation of shoulder movements. A disturbing click may rarely be felt during shoulder movements. Its pathogenesis is not known exactly, however, it is thought that it may be caused by recurrent minor traumas which emerge in the scapular region due to lower end of scapula rubbing against thoracic wall. Most of the patients are in 5th and 6th decades, and it is 10 times more frequent in women than in men [11]. The most frequent tumor in our series is elastofibroma. All of the tumors were totally excised. Because of the fact that elastofibroma emerged in the opposite site in the course of follow-ups of one case, that lesion was excised, as well, two years later. Due to bilateral elastofibroma, both lesions were excised at the same time in one case. All of these patients were women. It is found out that the patients with elastofibroma dorsi which does not require complex resection and reconstruction have the highest percentage in this patient group in routine practice.
As a consequence, in this single-center study in a training and research hospital, the frequency of chest wall tumors was not so high and although the knowledge that more than half of the chest wall tumors are malignant most of the lesions were benign which were resected completely.
Declaration of conflicting interests
The author declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The author received no financial support.
Reference
1) Pairolero PC. Chest wall tumors. In: General Thoracic Surgery, Shields TW, LoCicero J, Ponn RB (eds) 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2000: 589-98.
2) Bagheri R, Haghi SZ, Kalantari MR, Attar AS, Salehi M, Tabari A et al. Primary malignant chest wall tumors: analysis of 40 patients. J Thorac Cardiovasc Surg 1993; 105: 89-96.
3) Akay H. Göğüs duvarı tümörleri. In: Göğüs cerrahisi, Ökten İ, Güngör A (eds) Ankara: Sim Matbacılık; 2003: 731-45.
4) Sayır F, Sehitogullari A, Kahraman A, Sertogullarindan B, Esen R, Cobanoglu U. Primer Toraks Duvarı Tümörlerinin Analizi. Van Tıp Dergisi 2012: 19: 55-9.
5) Burt M, Karpeh M. Medical tumors of the chest wall. J Thorac Cardiovasc Surg 1993; 105: 89-96.
6) Mullan CP, Madan R, Trotman-Dickenson B, Qian X, Jacobson FL, Hunsaker A. Radiology of the chest wall masses. AJR Am J Roentgenol 2011; 197: 460-70.
7) Sabanathan S, Shah R, Mearns AJ. Surgical treatment of primary malignant chest wall tumours. Eur J Cardiothorac Surg 1997; 11: 1011-6.
8) Hasse J. Surgery for primary, invasive and metastatic malignancy of the chest wall. Eur J Cardiothorac Surg 1991; 5: 346-51.
9) Martini N, Huvos A, Smith J, Beatie EJ. Primary malignant tumors of the sternum. Surg Gynecol Obstet 1974; 138: 391-5.






