2Department of Anesthesia and Reanimation, Yedikule Chest Diseases and Thoracic Surgery Training and Research Hospital, Istanbul, Turkey DOI : 10.26663/cts.2024.021
Summary
For many years, pneumonectomy was considered the only type of surgery, especially for centrally located or locally advanced lung tumors. Bronchoplastic resections such as bronchial sleeve lobectomy have been developed as an alternative to pneumonectomy, which is a radical resection with parenchymasparing properties. As a result of the increasing use of video-assisted thoracoscopic surgery and the adaptation of extended resections to it, the use of VATS in sleeve resections has become widespread.Introduction
Sleeve resections are a specialized surgical method to preserve lung tissue in the treatment of benign or malignant lung pathologies. Bronchial sleeve lobectomy was first introduced as parenchyma-sparing surgery by Sir Clement Price-Thomas in 1947, and in 1954 Allison performed the first sleeve lobectomy for bronchogenic carcinoma. The first case of VATS bronchial sleeve was reported by Santambrogio et al in 2002, while the first case of uniportal VATS sleeve lobectomy was added to the literature by Diego Gonzalez-Rivas et al in 2013 [1-3].Sleeve lobectomy is generally performed in approximately 5% of all lung resections. The basis of sleeve resection is bronchial sleeve resections. Bronchial sleeve resections are a form of bronchoplastic resection based on the removal of diseased bronchial tissue together with tumor-containing lung parenchyma and anastomosis of intact bronchial orifices. Bronchial sleeve resections are performed as alternative operations to pneumonectomy in benign diseases as well as malignancies in order to preserve intact lung parenchyma. Segmenter resection, reconstruction, or angioplasty interventions can be performed on the pulmonary artery with or without bronchial sleeve resection. It is usually applied in three ways: 1st sleeve lobectomy (bronchial sleeve), 2nd vascular sleeve resection and 3rd sleeve pneumonectomy [1,4].
Sleeve lobectomies are mostly performed for tumors located at the mouth of the lobe bronchus and progressing to the main bronchus. Although bronchoplastic techniques are applicable to both main bronchi and all five major lobe bronchi, sleeve lobectomy is mostly performed in right or left upper lobe endobronchial tumors. Sleeve right upper lobectomy is the most commonly performed sleeve resection with a rate of approximately 70%. Sleeve lobectomy is less commonly performed when a lymph node invades the bronchial wall. The indication for sleeve resection with this type of lymph node involvement was seen in 21% of patients in the study conducted by Deslauriers et al in 1993 [5,6].
During surgery, after providing a suitable field of view and revealing the vascular and bronchial structures, solid margins on the bronchus should be determined and an incision should be made with a scalpel perpendicular to the long axis. The frozen section should be performed from the distal and proximal bronchial surgical margins and surgical margins should be expanded in case of tumor. Similarly, when excessive tension or impropriety in the anastomosis cannot be eliminated by various maneuvers, resection should be completed with pneumonectomy if necessary, considering the consequences of anastomotic leakage. In the anastomosis, care should be taken to ensure that the cartilage and membranous faces are opposite. The anastomosis can be completed with continuous suturing technique, separated suturing technique, or both techniques together [7-9].
Sleeve right upper lobectomy
Sleeve right upper lobectomy is the most commonly
performed sleeve lobectomy. In the surgical technique,
bronchial structures are exposed just below the azygos
vein for sleeve lobectomy after dividing the arterial and
venous branches of the right upper lobe and completing
the fissure. If necessary, the azygos vein can be divided
to provide better working space. Extensive radical dissection
in the peribronchial area may lead to disruption
of the vascular supply and ischemia of the anastomosis,
so it is recommended to keep the peribronchial dissection
minimal in these areas.
For sleeve right upper lobectomy after dissection, the right main bronchus is divided with a scalpel proximal to the upper lobe bronchial outlet. Similarly, the right intermediate bronchus is divided with a scalpel proximal to the middle lobe bronchial outlet. During bronchial division, the incision should be made in a smooth way perpendicular to the lumen. After the bronchial divisions are completed, the right upper lobe piece is taken out and subjected to frozen examination in both bronchial surgical margins. If the tumor is detected in the margin examination, the margins should be moved further by making a new incision from the bronchial stump or further resections such as pneumonectomy should be performed. In anastomosis, care should be taken to ensure that the membranous and cartilage faces face each other at both ends. Another important point is not to allow tension to build up at the anastomosis, which can be achieved by releasing the pulmonary ligament, pericardial, or hilar release maneuvers to increase lung mobilization. When there is a diameter mismatch between the right main bronchus and the intermediate bronchus, a telescopic anastomosis in which the distal end passes into the proximal end can also be performed. Single suture application, continuous suture technique, or both of these techniques can be used to complete the anastomosis. Absorbable or non-absorbable sutures can be preferred as material (Figure 1). After the anastomosis is completed, ventilation is provided with a pressure of 25-30 cm H2O, air leakage control is applied from the anastomosis. Supporting the anastomosis with structures such as intercostal muscle flap, pleura or thymic fatty tissue can be applied [10,11].
 Click Here to Zoom |
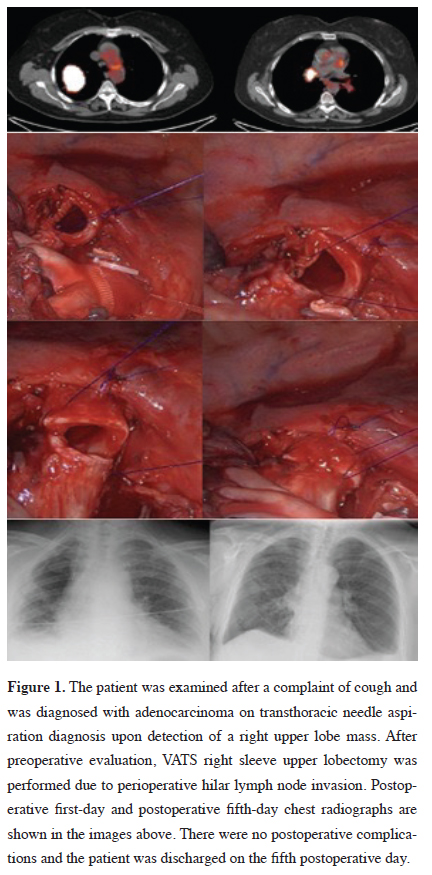
Figure 1: The patient was examined after a complaint of cough and was diagnosed with adenocarcinoma on transthoracic needle aspiration diagnosis upon detection of a right upper lobe mass. After preoperative evaluation, VATS right sleeve upper lobectomy was performed due to perioperative hilar lymph node invasion. Postoperative first-day and postoperative fifth-day chest radiographs are shown in the images above. There were no postoperative complications and the patient was discharged on the fifth postoperative day. |
Sleeve left upper lobectomy
Left-sided resections are more difficult because of the
narrow vision. The aortic arch and left pulmonary artery
limit access to the left main bronchus. There is also a
risk of injury to the left recurrent nerve that travels under
the arcus aorta.
In sleeve left upper lobectomy, the bronchial area is exposed by tilting the lung anteriorly after dividing the vascular structures extending to the upper lobe. At this stage, rotation, and lateral exclusion of the interlobar pulmonary artery will widen the visual angle. For sleeve left upper lobectomy, bronchial incisions are made in the left main bronchus and proximal to the left lower lobe superior segment outlet. The left lower lobe bronchus is anastomosed to the left main bronchus after verification of clean surgical margins by excising the upper lobe bronchus. Due to the length of the left main bronchus, the proximal bronchial incision can be made higher up. However, the anastomosis becomes technically difficult as you go proximally, especially because the arcus aorta restricts vision. Mobilization and careful retraction of the arcus aorta can be performed to provide additional visualization. Hilar release can be performed by dividing the pulmonary ligament and making a 'U' shaped pericardial incision at the level of the inferior pulmonary vein to reduce the tension in the anastomosis.
A centrally located tumor requiring sleeve lobectomy may also invade the pulmonary artery and arterial reconstruction may be required. This type of bronchial and vascular reconstruction is most commonly seen in sleeve left upper lobectomy among sleeve lobectomies [10-12].
Other sleeve resections have the same technical characteristics as thoracotomy in accordance with oncological principles.
Tips for VATS sleeve surgery
Different methods such as uniportal, two or three ports
can be used in VATS sleeve resections. It is important
to choose the optimal method for the primary surgeon's
preferred field of view and instrument use. In our clinic,
we use a biportal approach with a 4 cm utility incision
in the fourth or fifth intercostal space in the mid-axillary
region and a camera port in the seventh intercostal space.
Although we use a biportal approach during the division
of vascular structures, we switch to a uniportal approach
when bronchial anastomosis is started in upper sleeve
lobectomies. The assistant stands posterior to the patient
while holding the camera posterior to the uniportal
incision. The interlobar pulmonary artery is turned total
and suspended with silk or nylon tape and pulled away
from the anastomosis line with a clamp from the camera
port to facilitate visualization of the anastomosis area.
Anastomosis is started from the farthest corner of the
posterior area with a 3/0 polypropylene suture. Passing
the first suture inside to out proximal to the anastomosis
line allows the surgeon to work forehand. The side of
the first suture thread to be suspended is passed through
the chest wall and suspended (Figure 2). This prevents
tangling of the anastomotic suture threads. Among the
recommendations, the fact that the camera assistant is a
person who can perform VATS lobectomy contributes
significantly to shortening the operation time.
 Click Here to Zoom |
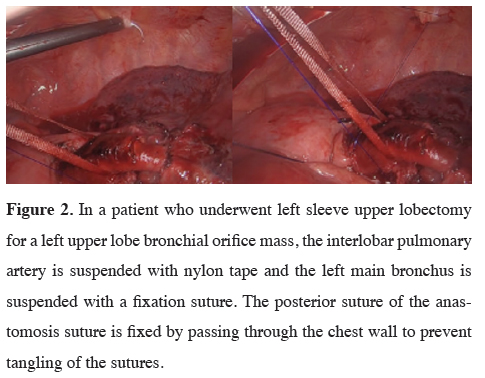
Figure 2: In a patient who underwent left sleeve upper lobectomy for a left upper lobe bronchial orifice mass, the interlobar pulmonary artery is suspended with nylon tape and the left main bronchus is suspended with a fixation suture. The posterior suture of the anastomosis suture is fixed by passing through the chest wall to prevent tangling of the sutures. |
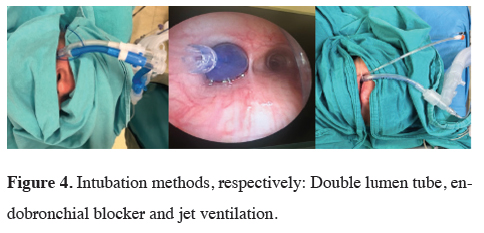
Anesthesia in VATS sleeve surgery
In our clinical practice, we use a double-lumen endobronchial
tube, bronchial blocker, or jet ventilation under
general anesthesia in patients scheduled for sleeve
bronchial surgery (Figures 3,4). Although a double-lumen
endobronchial tube is our most preferred method
for single lung ventilation, we also frequently use bronchial
blocker with fiberoptic bronchoscopy when the
patient's airway is difficult. Jet ventilation is performed
with a tritube and an accompanying bronchial blocker
in cases of carinal sleeve. As a result of working in harmony
with the surgical team in case of implementation
difficulties, we provide a sterile breathing circuit in the
surgical field and ventilation continuation with an endotracheal
tube advanced distal to the anastomosis.
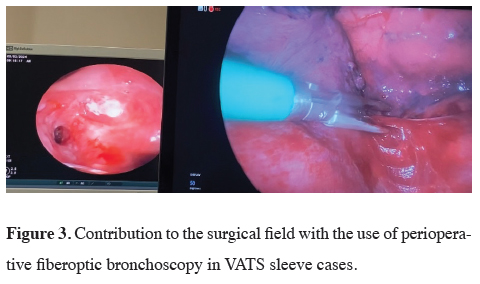
Thanks to our experience in the use of fiberoptic bronchoscopy, we often have no difficulty in single lung ventilation, but we assist the surgical team in endobronchial imaging when used intraoperatively (Figure 3). In sleeve surgeries, providing comfort to the surgical field shortens the operation time and reduces the complications that may occur in the patient.
 Click Here to Zoom |
Figure 3: Contribution to the surgical field with the use of perioperative fiberoptic bronchoscopy in VATS sleeve cases. |
 Click Here to Zoom |
Figure 4: Intubation methods, respectively: Double lumen tube, endobronchial blocker and jet ventilation. |
The most mortal complication that may occur in sleeve resections is early anastomotic line separation. Bronchovascular fistula, bronchial stenosis and atelectasis are other major complications. In Tedder's publication in which 1125 cases of sleeve lobectomy performed in different centers were examined, the rate of pneumonia was 6.7% and the rate of atelectasis was 5.4%. The development of pneumonia and atelectasis has been associated with loss of ciliary function and mucosal damage at the anastomotic line. To minimize these complications, bronchoscopy in the operating room immediately after surgery is recommended. Indications for bronchoscopy in the postoperative period include persistent wheezing, the appearance of atelectasis or lobar consolidation on chest radiography, or prolonged air leakage exceeding seven days [8,13].
Anastomotic complications, especially bronchial stenosis, bronchovascular and bronchopleural fistula, can be reduced by using of living tissues between bronchial and vascular structures and supporting the anastomosis with anatomical structures. Among these complications, bronchopleural fistula has been reported between 1-14% after sleeve resections [10]. Since hemoptysis in the postoperative period after sleeve lobectomy may be a sign of a bronchovascular fistula, urgent bronchoscopic evaluation is required [13].
In the study by Gao et al in 2019, after 54 VATS sleeve and 94 thoracotomy sleeve cases were evaluated with propensity score, 39 cases were included in each group. The results of the evaluation showed that VATS resulted in significantly less blood loss, chest tube and hospital stay. The mean operation time was 300 minutes with VATS and 221 minutes with thoracotomy. A decrease in economic costs was found in VATS resections. No difference in postoperative complications was observed between both groups [14].
Deng et al performed a meta-analysis of 5 studies in which VATS sleeve and thoracotomy sleeve were performed after evaluation of the literature. A total of 436 cases were evaluated; the VATS sleeve group had less blood loss, longer operation time, and shorter hospital stays. Postoperative complications were 13.5% in the VATS group and 15.7% in the thoracotomy group with no significant difference. Three-year survival was 72.5% in the VATS group and 66.8% in the thoracotomy group and no significant difference was observed [15].
Huang et al analyzed 118 VATS bronchial sleeve cases. Three port approaches were used. Mean operation time was 124 (118-223) minutes and bronchial anastomosis time was 30 (15-42) minutes. The mean postoperative drainage period was 5 (3-8) days. In a single case, bronchial leakage was detected on the 12th postoperative day and the patient died with massive hemoptysis [16].
Soultanis et al analysed 79 cases of uniportal sleeve lobectomy. Mean operation time was 180 (maximum 390) minutes and blood loss was 100 (maximum 1480) ml. The postoperative drainage period was determined as 3.8 days. In the series, thoracotomy was performed in one case due to massive hemorrhage and revision was performed in one postoperative case due to hemorrhage. Atelectasis, pneumonia, and anastomosis-related complications were not detected. One month later, all patients were evaluated by bronchoscopy and anastomosis lines were found to be normal [17].
In the study by Huang et al, 13 cases of thoracoscopic double sleeve were analyzed. Mean operation time was 264 (218-330) minutes and pulmonary artery clamping time was 72 (44-143) minutes. The mean pulmonary artery anastomosis time was 45 (26-75) and the mean bronchial anastomosis time was 31 (15-50) minutes. In the series, 30-day mortality was not detected and postoperative pneumonia was observed in one patient [18].
In our clinic, the number of VATS sleeve resection cases has increased in the last two years. With our experience, we found no difference in operative time between thoracotomy and VATS cases. We observed a shorter duration of tube drainage and hospitalization in VATS cases.
In conclusion, with the increasing experience in minimally invasive approaches, thoracoscopic surgery has started to be used at higher rates. VATS sleeve resections have started to be performed with the developing endoscopic instruments and surgical techniques. VATS without compromising oncological principles resulted in less surgical trauma and faster postoperative recovery. Considering these reasons, we recommend VATS sleeve lobectomy in clinics with high thoracoscopic experience.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect
to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research
and/or authorship of this article.
Authors’ contribution
MÜ,EG,MM: conceived and designed the review, cowrote
the paper, collected the clinical data. The authors
discussed the paper under the literature data together
and constituted the final manuscript.
Reference
1) Santambrogio L, Cioffi U, De Simone M, Rosso L, Ferrero
S, Giunta A. Video-Assisted Sleeve Lobectomy for Mucoepidermoid
Carcinoma of the Left Lower Lobar Bronchus. Chest
2002; 121: 635-6.
2) Gonzalez-Rivas D, Fernandez R, Fieira E, Rellan L. Uniportal
video-assisted thoracoscopic bronchial sleeve lobectomy: First
report. J Thorac Cardiovasc Surg 2013; 145: 1676-7.
3) Nykamp V. Surgical treatment of non-small cell lung cancer. S
D Med 2010; Spec No: 57-9.
4) Ponn Rb, LoCicero III J, Daly BDT. Surgical Treatment of Non-
Small Cell Lung Cancer. In: Shields TW, LoCicero III J, Ponn Rb,
Rusch VW, editors. General Thoracic Surgery. Lippincott Williams
and Wilkins, Philedelphia, USA, 6th ed, 2005; 1549-87.
5) Deslauriers J, Mehran RJ, Guimont C, Brisson J. Staging and
management of lung cancer: Sleeve resection. World J Surg
1993; 17: 712-8.
6) Icard P, Regnard J, Guibert L, Magdeleinat P, Jauffret B, Levasseur
P. Survival and prognostic factors in patients undergoing
parenchymal saving bronchoplastic operation for primary lung
cancer: a series of 110 consecutive cases. Eur J Cardiothorac
Surg 1999; 15: 426-32.
7) Hollaus PH, Janakiev D, Pridun NS. Telescope anastomosis in
bronchial sleeve resections with high-caliber mismatch. Ann
Thorac Surg 2001; 72: 357-61.
8) Kutlu CA, Goldstraw P. Tracheobronchial sleeve resection with
the use of a continuous anastomosis: Results of one hundred consecutive
cases. J Thorac Cardiovasc Surg 1999; 117: 1112-7.
9) Deslauriers J, Gaulin P, Beaulieu M, Piraux M, Bernier R,
Cormier Y. Long-term clinical and functional results of sleeve
lobectomy for primary lung cancer. J Thorac Cardiovasc Surg
1986; 92: 871-9.
10) Nesbitt JC, Wind GG. Thoracic surgical oncology: exposures
and techniques. 2003: Lippincott Williams and Wilkins (ISBN:
0683064290).
11) Kaiser LR, Jamieson G. Operative Thoracic Surgery Fifth Edition.
Vol. 7. 2006: CRC Press (ISBN: 978144411357).
12) Toker A, Tanju S. A patch plasty to inferior pulmonary vein:
when more release is needed. Eur J Cardiothorac Surg 2010;
38:234.
13) Faber LP. General Thoracic Surgery. J Am Coll Surg 1998;
186: 162-72.
14) Gao H, Jiang Z, Gong L, Mai K, Ren P, Yu ZT et al. Video-Assisted
Vs Thoracotomy Sleeve Lobectomy for Lung Cancer: A Propensity
Matched Analysis. Ann Thorac Surg 2019; 108: 1072-9.
15) Deng H, Qiu X, Zhu D, Tang X, Zhou Q. Video-Assisted Thoracoscopic
Sleeve Lobectomy for Centrally Located Non-small
Cell Lung Cancer: A Meta-analysis. World J Surg 2021; 45:
897-906.
16) 16. Huang J, Li S, Hao Z, Chen H, He J, XU X et al. Complete
video-assisted thoracoscopic surgery (VATS) bronchial sleeve
lobectomy. J Thorac Dis 2016; 8: 553-74.