Summary
Pediatric esophageal emergency pathologies such as esophageal perforation, corrosive esophagitis and esophageal foreign bodies are complex clinical conditions. Most important point of these pathologies are taking a careful history, focused physical examination, and screening laboratory tests. The mortality and morbidity rates are depending on the time between diagnosis and treatment. The aim of the study is to develop practical algorithms in diagnosis and treatment from the perspective of thoracic surgery that guides preclinical and specialized clinical physicians in the treatment of pediatric esophageal emergency pathologies.Introduction
Pediatric esophageal emergency pathologies, consisting of esophageal perforation, corrosive esophagitis, and esophageal foreign bodies, are conditions that have extremely dangerous consequences in terms of both morbidity and mortality. Treatment options should be determined and applied specifically to the patient, diagnosis, severity of the condition, and other medical conditions [1]. The Most important point of these pathologies are taking a careful history, focused physical examination, and screening laboratory tests. And also, the most important point determining the mortality and morbidity rate is the time between diagnosis and treatment [1,2].The review aims to develop an algorithm that guides preclinical and specialized clinical physicians in the treatment of pediatric esophageal emergency pathologies. We believe that this will enable the effective and prompt identification and treatment of rare conditions associated with pediatric esophageal issues.
Corrosive esophagitis
Corrosive injuries result from the ingestion of caustic
or corrosive substances through the oral route and can
lead to erosive esophagitis with strictures in the chronic
phase. Although acknowledging a continuous decrease
in developed nations, the annual incidence is estimated
to be between 5,000 and 15,000 cases globally. The
clinical manifestations of caustic ingestion in children
can range from no injury to fatal outcomes. It's important
to note that nearly all corrosive injuries in children
are accidental [1].
Caustic injuries are more commonly observed in countries where preventive measures are lacking, often due to social, economic, and educational factors. Approximately half to 80% of these injuries occur in children. While preventive measures have successfully reduced caustic injuries in many countries, there is still a need for the realization of this goal in numerous developing nations. The ingestion of highly alkaline or acidic substances remains a significant cause of morbidity and mortality globally, particularly in developing regions [1,2].
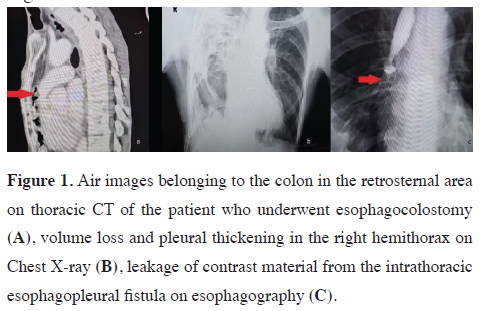
Accidental caustic ingestion poses the highest risk for children under 5 years old due to their curiosity and welldeveloped skills to find and drink potentially toxic substances. Risk factors for corrosive injuries in this group include male gender, attention deficit hyperactivity disorder, lower parental education, young maternal age, low socioeconomic status, and living in rural areas (Figure 1).
 Click Here to Zoom |
Figure 1: Air images belonging to the colon in the retrosternal area on thoracic CT of the patient who underwent esophagocolostomy (A), volume loss and pleural thickening in the right hemithorax on Chest X-ray (B), leakage of contrast material from the intrathoracic esophagopleural fistula on esophagography (C). |
Approximately 20-40% of individuals who ingest caustic substances develop erosive esophagitis. The severity of corrosive esophagitis depends on factors such as the amount, concentration, pH, and duration of contact with the caustic agents [2]. Risk factors of caustic ingestion in children are shown in table 1.
Table 1. Risk factors of caustic ingestion in children [3-11].
Both alkali (pH; 4-11.5) and acid (pH; less than 2) substances can cause significant burns to the cheeks, mouth, oropharynx, esophagus, and stomach, and rarely to the duodenum, as well as the airway. Serious long-term gastrointestinal morbidity occurs in 7-25% of cases, whether or not a stricture is present. Esophageal injury, with or without stricture, results in fibrosis, and abnormal esophageal peristalsis may lead to dysphagia dysphagia [12-14].
In children ingesting caustic substances, the development of strictures ranges from 10% to 75%, depending on the concentration involved. Alkaline ingestion is more prevalent in Africa, the Americas, and Oceania, while acid ingestion is more common in Europe and Asia for children. Despite the higher prevalence of acid ingestion in certain regions, stricture formation is more commonly associated with alkaline ingestion. The incidence of stricture formation after acid ingestion in children is reported to be in the range of 2.9% to 15.3% [15].
The mechanism of tissue damage caused by caustic substances depends on their pH. Acidic substances cause coagulation necrosis due to protein denaturation, while alkaline substances cause liquefaction necrosis by destroying cell architecture. As a result, acids typically cause superficial injuries, whereas alkalis cause deeper injuries. While it's not an absolute rule, acidic substances often lead to long-segment gastrointestinal injuries, while alkaline substances tend to cause more limited injuries in the esophagus [16,17].
In the first week after caustic ingestion, mucosal damage and bacterial invasion become apparent. Full-thickness injuries during this period can lead to perforation. Esophageal remodeling begins around the start of the second week, and as a result, endoscopy is typically not performed between 5 and 15 days after the injury. Scar retraction starts by the third week, driven by fibroblast proliferation. Mucosal re-epithelialization begins during this period and is usually completed by the sixth week after the injury. This timeline highlights the dynamic process of tissue response and repair following caustic ingestion [2].
Presentation and emergency department treatment
Patients typically present to the emergency department
following the ingestion of a caustic substance. If the ingested
substance is solid, it often causes oropharyngeal
injuries, while liquids can result in burns to the esophagus.
The presence of oral injury generally indicates esophageal
injury as well. However, the absence of oral injury does
not rule out esophageal injury. In patients with caustic injury
but no oral lesions, approximately 1% may develop
esophageal strictures in the chronic period [13].
Many patients are initially asymptomatic, but despite this, esophageal injury can be detected in a significant number through endoscopy. In 6-18% of patients, oropharyngeal and laryngeal injuries may lead to symptoms such as hoarseness, stridor, dyspnea, tachypnea, or wheezing. Severe injuries can result in transmural necrosis, perforation, and mediastinitis, although this is very rare. If a patient develops fever, tachycardia, severe retrosternal or abdominal pain, hemorrhage, sepsis, or organ dysfunction develop in a patient, urgent surgical intervention may be necessary.
The presence of symptoms like drooling, dysphagia, and epigastric pain may indicate a possible gastrointestinal injury. Severe injuries are suggested by vomiting and hematemesis. If a patient shows signs of gastrointestinal injury, such as drooling, dysphagia, and epigastric pain, or if severe symptoms like vomiting and hematemesis are present, it indicates a more severe gastrointestinal injury, and prompt medical attention is crucial.
Treatment
Firstly, it is necessary to determine the type and
quantity of the ingested substance. The patient should
never be induced to vomit. Neutral liquid such as water
or milk can’t be given due to concerns regarding potential
further harm from heat injury during the neutralization
process, diluting agents should be avoided, as they
pose safety risks. During this process, it is crucial to
start intravenous fluid infusion for the patient [18].
If there is an airway injury, respiratory stabilization (intubation and/or tracheostomy) should be prioritized, followed by an evaluation of the esophagus. Approximately 18% of patients may require emergency tracheostomy, and 1% may need chronic tracheostomy [19].
Although there is no evidence in the literature, broadspectrum antibiotic therapy can be initiated or prophylaxis can be applied before endoscopy due to the potential for perforation and other bacterial complications [20].
The first diagnostic test should be a posteroanterior chest X-ray. The X-ray is used to evaluate mediastinal widening, mediastinal emphysema, intra-abdominal free air, and pneumothorax. The gold standard for detecting esophageal injury is esophagogastroduodenoscopy. Endoscopic evaluation should be performed within the first 24-48 hours and under general anesthesia, as the risk of perforation is lowest during this period. Additionally, to minimize the risk of possible perforation, air insufflation during endoscopy should be minimal or avoided altogether. The endoscope should not be blindly advanced, and the procedure should be terminated if injury is observed. In cases of potential severe injury, careful nasogastric tube placement with endoscopic visualization should be performed to facilitate enteral nutrition and prevent gastric content treatment at the site of injury injury [21-23].
The Zargar et al classification system proposed in 1994 is commonly used to grade esophageal injuries in clinical practice [20,24] (Table 2). In the acute phase, the presence of perforation, its location, and dimensions, along with accompanying complications such as mediastinitis, pneumonia, etc., can be assessed using contrast swallow studies with water-soluble contrast (X-ray and/or CT). In the chronic phase (3-6 mounts later), potential stricture development, pyloric obstruction, or the evaluation of treatment response after dilation following stricture development can also be assessed using these imaging techniques [25,26].
Table 2. Classification in caustic injuries.
Preventive treatment
PPIs or histamine H2-receptor antagonists: To reduce
stomach acid.
Oral nystatin suspension: To prevent endogenous fungal overgrowth, exacerbation of stricture formation, and colonization of the nasogastric tube. It should be used for 3 weeks.
Nasogastric tube placement: For early enteral feeding.
Steroids: The use of high-dose methylprednisolone (1 g/1.73m2 daily for 3 days) decreases stricture formation. However, the use of steroids in caustic esophagitis is controversial. It has been determined that patients with first or third-degree esophageal injuries do not benefit from steroid use. Still, steroids can prevent stricture formation in patients with second-degree caustic esophagitis [1,28-30].
Treatment of long-term complications
Esophageal strictures
Esophageal strictures in children are most commonly
caused by caustic injuries. Strictures are most commonly
determined in the cervical and middle thoracic
esophagus, with an incidence ranging between 2% and
49%. It has been demonstrated in the study conducted
by Nunes et al that severe endoscopic lesions, involvement
of the entire length of the esophagus, hematemesis,
and increased serum lactic dehydrogenase represent
risk factors for the development of fibrotic strictures induced
by caustic ingestion. Serial stricture dilation is the
mainstay of therapy for esophageal strictures. However,
esophageal replacement is the most suitable treatment choice for long and/or refractory strictures [1,27-29].
If patients experience symptoms of dysphagia, and a stricture is identified endoscopically, a dilation program can be initiated 3 weeks after the injury. The success rate of dilation is very high for non-lye strictures, strictures under 5 cm in length, strictures in the upper third of the esophagus, and in younger patients (under 8 years of age). Weekly to bi-weekly dilations over 3 months, with an average of 12 dilations, are required for strictures due to corrosive injuries. The perforation rate of dilation ranges from 0.4% to 17.4% [1,5,8].
Esophageal replacement surgery is required in 18% of patients who experience perforation during dilation, and the mortality rate increases to 18% in such cases [9]. Some reports suggest that the frequency and necessity of dilatation may decrease with the injection of steroids and/or mitomycin into the stricture area, although there are limited pediatric reports on this approach [9].
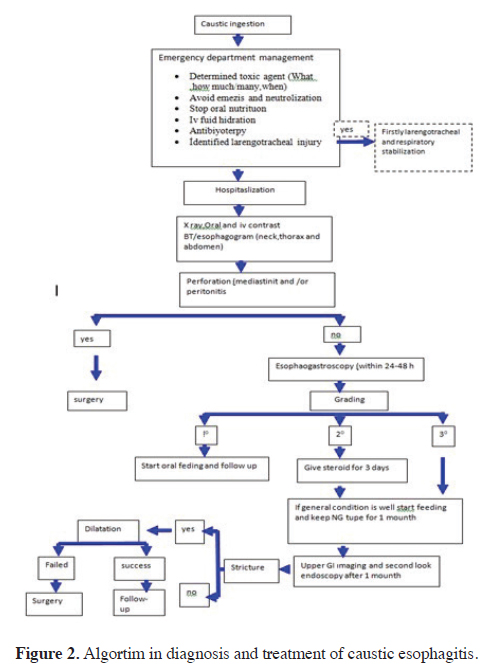
Stenting of esophageal strictures with double-tube polyamide and silicone, self-expanding covered metal (nitinol) stents, or polydioxanone absorbable stents may be considered as a good alternative to serial/multiple dilations or surgery. However, there are challenges associated with stenting in pediatric cases, including the lack of stents suitable for children, high costs, stent migration, and the potential for secondary stenosis due to granulation tissue in the proximal and distal parts of the stent [1,30]. The diagnosis and treatment algorithm for caustic injuries is prepared based on the literature data and our clinical experience (Figure 2).
 Click Here to Zoom |
Figure 2: Algortim in diagnosis and treatment of caustic esophagitis. |
Esophageal perforation
Esophageal perforation (EP) is rare in the neonatal population,
with an incidence reported as 0.006% in large
series [31]. The first spontaneous esophageal perforation
due to the esophageal web was reported by James
Fryfogel in 1952. Additionally, the first iatrogenic
esophageal perforation was reported during stiff rubber
catheter suctioning in a 28-day-old infant in 1961 [32].
The esophagus is highly susceptible to trauma and injury
due to the absence of supporting tissue around its
serosal layer. Once an injury occurs, bacterial spread
and the inflammatory response can rapidly and easily
lead to complications. Polymicrobial flora dominated
by anaerobes, along with gastrointestinal content, can
spread into the mediastinum, resulting in conditions
such as mediastinitis, empyema, abscesses, sepsis, and
multiple organ dysfunction syndrome, all of which have
high mortality and morbidity rates [33].
Despite improvements in post-operative intensive care unit management, antibiotic therapy, and advances in enteral and parenteral nutrition, esophageal perforation remains a life-threatening condition. The mortality rate has recently been reported as 28%. It's crucial to note that after the diagnosis of perforation, mortality doubles for every 24 hours that pass without treatment [34].
Etiology
Esophageal perforation can occur due to various reasons,
with endoscopic stricture dilation and nasogastric
tube insertion. The table given below outlines the most
common causes of esophageal perforation (Table 3). In
children, the most frequent causes include iatrogenic
traumas, lye burns or chemical burns, direct and indirect
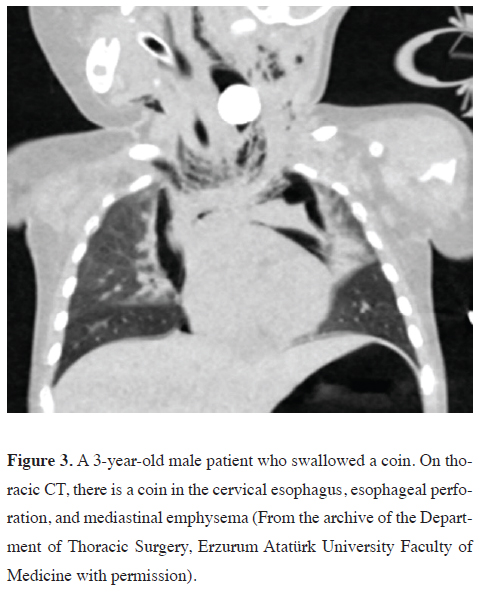
trauma, ingestion of foreign objects (Figure 3), operative
trauma, and idiopathic causes.
Table 3. Reasons of esophageal perforation.
 Click Here to Zoom |
Figure 3: A 3-year-old male patient who swallowed a coin. On thoracic CT, there is a coin in the cervical esophagus, esophageal perforation, and mediastinal emphysema (From the archive of the Department of Thoracic Surgery, Erzurum Atatürk University Faculty of Medicine with permission). |
Clinical presentation
Symptoms of esophageal perforation can vary based
on the cause, location of the perforation, and the time
between diagnosis and treatment. However, the most
common symptoms include dysphagia (60%), dyspnea,
and fever. In cervical perforations, symptoms tend to
be less severe as the spread of contamination from the
retroesophageal space to the mediastinum is slow. On
the other hand, thoracic perforations lead to rapid contamination
of the mediastinum, resulting in symptoms
of systemic sepsis and shock within 24 hours. Gastric
contents and bacterial flora from the gastrointestinal
tract infiltrate the mediastinum, causing severe necrotizing
mediastinitis. Negative intrathoracic pressure can
worsen pleural rupture, leading to pleural effusion and
pneumothorax. In intra-abdominal esophageal perforations,
peritonitis is a common presentation, and symptoms
may appear within a few hours [35,36].
In neonates, symptoms of esophageal perforation may include hypersalivation with choking, coughing, or cyanosis during feeding. Neonates might also present with a pneumothorax. Fever is often observed in the first 24 hours after challenging endotracheal intubation or nasogastric tube insertion [34]. Symptoms according to the location of the perforation are shown in table 4.
Table 4. Symptoms according to the location of the perforation.
Diagnosis
The diagnosis of esophageal perforation can be made
through various methods. Although it is normal in 12-
33% of patients, neck and chest X-rays should be the
first diagnostic test in patients with suspected esophageal
perforation. A plain X-ray can demonstrate the
localization of the enterogastric tube, pneumothorax,
pleural effusion, pneumomediastinum, and mediastinal
enlargement. In a patient with findings suggestive of
esophageal perforation on X-ray, the diagnosis is confirmed
through a contrast-enhanced esophagogram and
endoscopy. These examinations help to determine the
location and size of the perforation, with a reported sensitivity
of 100% in the literature [37].
In clinical practice, especially in infants, endoscopy is not recommended due to the difficulty of application and the risk of increasing the size of the perforation [34,36]. Esophagography findings in esophageal perforation include;
• A collection of contrast in a retropharyngeal pocket from a localized cervical leak.
• A submucosal perforation, appearing as a tract parallel and posterior to the esophageal column.
• Free perforation into the pleural space [32,35,36].
Chest computed tomography (CT) can be very useful in diagnosing esophageal perforation. In patients with esophageal perforation, CT may reveal air and fluid in the extraesophageal area in the mediastinum, esophageal wall thickening, and mediastinal or para mediastinal air-fluid collection, abscess cavities in the pleural space or mediastinum [37-40].
Treatment
Surgical treatment
Surgical indications in esophageal perforation (EP) include
clinical deterioration, persistence of a leak despite treatment,
massive intrapleural or retropharyngeal spillage,
sepsis, mediastinitis, abscess formation in pleural and mediastinal
spaces, or even massive or persistent leaks [41].
The most important factor determining post-treatment mortality and morbidity is the time between diagnosis and treatment. The main principles of surgical treatment include the debridement of devitalized tissue from the perforation site, adequate pleural drainage, and elimination of the contamination source. After debriding the devitalized tissue from the perforation site, the muscular layer and mucosa must be closed with absorbable interrupted sutures. The primary repair site should be reinforced using intercostal, omental, pericardial fat pad, diaphragmatic pedicle graft, rhomboid muscle, latissimus dorsi muscle, and intercostal muscle or pleural flaps. Surgical approaches according to the perforation site are shown in the table 5 [34 -38].
Table 5. Surgical approaches according to the perforation site.
Esophagectomy may be necessary in cases of severe necrosis and multiple injuries due to caustic esophagitis, severe mediastinal contamination, and failure of primary repair. Diversion with cervical esophagostomy might be required if the esophagus is too friable, the patient remains unstable, the repair is not possible due to the size of the defect or friability of surrounding tissue, or widespread mediastinitis prevents primary repair during the initial procedure [34-38].
Recently, minimally invasive surgery has been employed in esophageal perforation. However, there are only a small amount of patient series reported in the literature that were treated with thoracoscopic drainage of the mediastinum, and no mortalities were reported in these series [32,34,36,37].
Non-surgical treatment
In the literature, the total mortality of non-operative
treatment in esophageal perforation (EP) is reported to
be 18%. This rate is lower than other treatments for EP
in childhood compared to adults. Non-operative treatment
consists of antibiotics that cover both anaerobes
and aerobes, adequate fluid resuscitation, stopping oral
intake and starting parenteral nutrition, instituting gastric
drainage via gastrostomy or nasogastric tube, and placing
a thoracostomy tube. Oral nutritional intake is stopped
to decrease further contamination of the wound and allow
for esophageal healing [38,39,41,42].If enteral tubes
can be safely passed under fluoroscopic guidance, enteral nutrition should be continued. If not, total parenteral nutrition
(TPN) should be reserved. Oral feeds can be resumed
after an esophagram shows no further leak. It is
reported that the average interval to the first esophagram
after diagnosis is 7 days, and the average interval to the
initiation of oral feeds is 11.5 days [38].
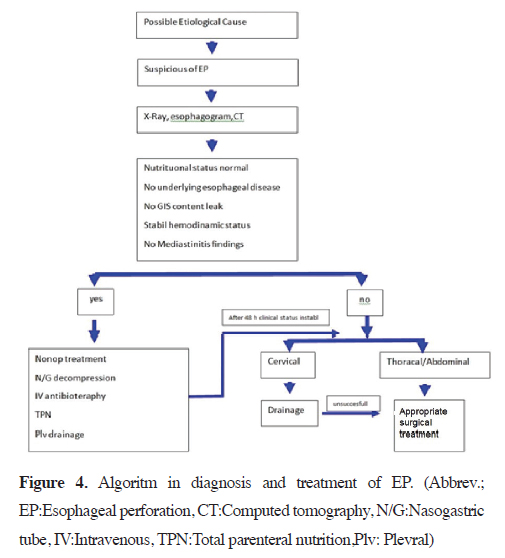
Systemic infection and sepsis progression can be prevented by using broad-spectrum antibiotics that generally cover gram-negative and anaerobic spectra. Typically, antibiotic therapy consists of piperacillin/tazobactam, vancomycin, and gentamicin, along with antifungal agents. The treatment is continued for 7–14 days from the time of perforation diagnosis. If the leak due to perforation persists on esophagography, antibiotic therapy must be continued for an additional 7 days. If pneumothorax and/or pleural effusion are present, drainage must be provided by tube thoracostomy. Pneumothorax has been reported in 32% of patients with esophageal perforation. It is usually seen in the right hemithorax and can be treated with a chest tube or small pigtail catheters [42]. The diagnosis and treatment algorithm for esophageal perforation is prepared based on the literature data and our clinical experience is shown in figure 4.
 Click Here to Zoom |
Figure 4: Algoritm in diagnosis and treatment of EP. (Abbrev.; EP:Esophageal perforation, CT:Computed tomography, N/G:Nasogastric tube, IV:Intravenous, TPN:Total parenteral nutrition,Plv: Plevral) |
Foreign Bodies
Aerodigestive foreign bodies are one of the most important
morbidity and mortality reasons in infants and
children. The aspiration of foreign bodies usually cause a chronic lung infection. and most of the patients admitted
to hospital for this reason. Prevalence of foreign
body ingestion is reported as 17.9 / 10,000 children who
are applied in U.S. emergency departments [43]. The
most commonly seen FBs are coins, toys, batteries, and
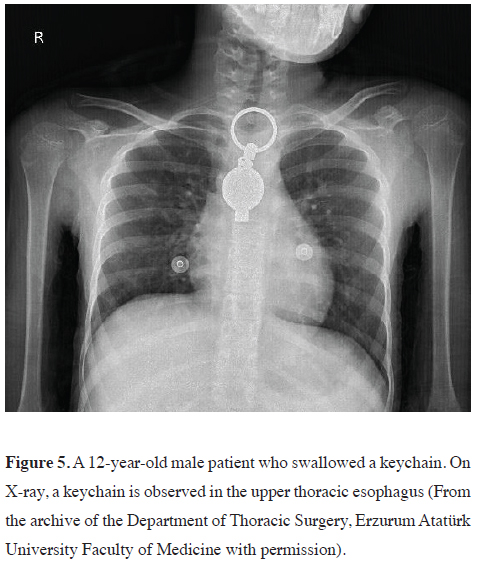
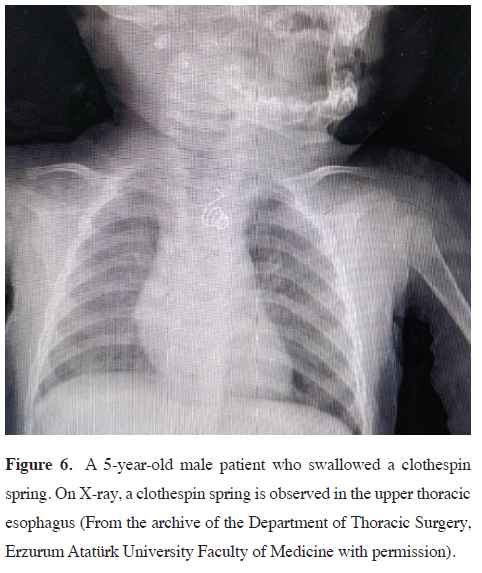
high-powered magnets [44] (Figures 5,6).
 Click Here to Zoom |
Figure 5: A 12-year-old male patient who swallowed a keychain. On X-ray, a keychain is observed in the upper thoracic esophagus (From the archive of the Department of Thoracic Surgery, Erzurum Atatürk University Faculty of Medicine with permission). |
 Click Here to Zoom |
Figure 6: A 5-year-old male patient who swallowed a clothespin spring. On X-ray, a clothespin spring is observed in the upper thoracic esophagus (From the archive of the Department of Thoracic Surgery, Erzurum Atatürk University Faculty of Medicine with permission). |
While a lot of GIS foreign bodies pass spontaneously through the gastrointestinal (GI) tract, 40% of esophageal FBs are asymptomatic. For this reason, diagnosis and treatment are delayed and complications that may be mortal and morbid (such as oesophageal perforation, periesophagitis, and mediastinal infection) may appear [45]. The most common symptoms are dysphagia, vomiting, drooling, feeding refusal, and neck and chest pain. According to the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition guidelines, Large FBs even compressing the airway may present with stridor, wheezing, and respiratory distress [46,47].
Because of cricopharyngeal area in the cervical esophagus is the narrowest part of the GIS tract, foreign bodies most commonly lodge in this area. If the FBs cross the cricopharyngeal area, these often pass through into the lower GIS tract and uneventfully are excreted from the intestine [48]. In radiopaque esophageal FBs, diagnostic procedures are firstly neck and chest PA and lateral X-rays even if, plain radiographs of the neck are normal, if a retained esophageal object is suspected by history, examination, or symptoms, an esophagram is needed [49,50].
Treatment
The most common treatment strategies for EFBs in
children are rigid oesophagoscopy and Foley catheter
balloon extraction. The use of a Foley catheter is used,
while the patient is maintained in the Trendelenburg
position. Complications of Foley catheter include perforation,
aspiration, and acute airway obstruction. The
Foley catheter is a safe, efficient, convenient, and practical
method for the treatment of children with coins
and other blunt EFBs, and it can significantly reduce the
need for endoscopy. Some meta-analyses showed that
no significant difference between rigid and flexible endoscopy
regarding efficacy and safety for the treatment
of esophageal foreign bodies [51,52]. However, rigid
oesophageal endoscopy under general anesthesia is recommended
in Children with EFBs to protect the airway.
Moreover, in literature, it is strongly recommended that
rigid endoscopy for children because it allows both the
use of optical forceps with a strong grasping ability for
EFBs and the positioning of sharp and pointed objects
inside the rigid endoscope the removal of oesophageal
button is extremely more important than other foreign
bodies. Because, these cause chemical corrosion, electrical
damage, thermal burns, and physical compression to the oesophageal mucosa. Oesophageal necrosis and
even life-threatening complications can occur within 2
hours after accidentally ingesting a button battery. For
this reason, in patients, emergency endoscopy and/or
operation should be performed without fasting or presurgical
preparation to reduce the corrosion of the oesophageal
mucosa [50-51].
Surgical intervention is indicated in cases of large, sharp-edged foreign bodies embedded in the mucosa that cannot be removed endoscopically and in the presence of perforation. In these situations, employing a surgical method (such as VATS and/or thoracotomy) for the removal of the foreign body would be the safest approach [50-51].
Complications
The complication rate of rigid endoscopy for either airway
or esophageal FB is low (0.2%-5%) and mortality
is rare (<0.1%). Postoperative complications increase
for children who present with a delay in diagnosis
greater than 24 hours. Delayed treatment or failed and/
or improper treatment can cause complications [51].
The factors of complications in EFB patients are complex.
It was shown that sharp foreign bodies and long
durations have a higher incidence of complications due
to mechanical damage to the mucosa. Risk factors for
complications in EFBs are the younger the child, the
long implication time of the foreign body, the sharp foreign
body, the location of the foreign body, and the presence
of fever or cough [49,50].
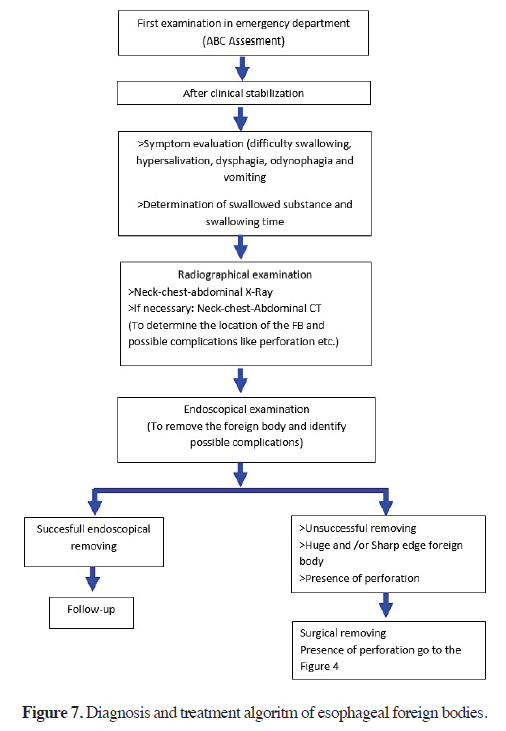
In young children, the lumen at the entrance of the esophagus is narrow and the oesophageal muscle layer is weak. For this reason, this patient’s esophagus is more vulnerable. Sharp foreign body may cause edema and inflammation of the oesophageal mucosa, and longterm compression of the oesophageal mucosa will cause ischaemic necrosis. Esophageal endoscopy performed in a narrow and oedematous oesophageal cavity also increases the risk of mechanical damage and mucosal tearing injury. After 24 h, complication risk significantly increases regardless of the type of foreign body and other factors. All of the esophageal foreign bodies must be removed within 24 h [47-52]. The diagnosis and treatment algorithm for esophageal foreign bodies is prepared based on the literature data and our clinical experience is shown in figure 7.
 Click Here to Zoom |
Figure 7: Diagnosis and treatment algoritm of esophageal foreign bodies. |
In conclusion, pediatric esophageal emergency pathologies are conditions that require long-term multidisciplinary follow-up due to their chronic complications. The most important aspects of managing these pathologies are taking a careful history, conducting a focused physical examination, and performing screening laboratory tests. By doing so, the development of chronic complications, which make treatment most challenging, can be prevented.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect
to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research
and/or authorship of this article.
Authors’ contribution
MGP,SG,Cİ,ATŞ,YB: conceived and designed the review,
co-wrote the paper, collected the clinical data.
The authors discussed the paper under the literature data together and constituted the final manuscript.
Acknowledgement
We would like to thank the Department of Thoracic Surgery
at Erzurum Atatürk University Faculty of Medicine
for figures 2, 3, and 4.
Reference
1) Huang YC, Ni YH, Lai HS, Chang MH. Corrosive esophagitis
in children. Pediatr Surg Int 2004; 20: 207-10.
2) De Jong AL, Macdonald R, Ein S, Forte V, Turner A. Corrosive
esophagitis in children: a 30-year review. Int J Pediatr Otorhinolaryngol
2001; 57:203-11.
3) Çakmak M, Göllü G, Boybeyi Ö, Küçük G, Sertçelik M, Günal
YD et al. Cognitive and behavioral characteristics of children
with caustic ingestion. J Pediatr Surg 2015; 50: 540-2.
4) Karaman İ, Koç O, Karaman A, Erdoğan D, Çavuşoğlu YH,
Afşarlar ÇE et al. Evaluation of 968 children with corrosive
substance ingestion. Indian J Crit Care Med 2015; 19: 714-8.
5) Mas E, Breton A, Lachaux A. Prise en charge des enfants après
ingestion de substances acides ou alcalines [Management of
caustic esophagitis in children]. Arch Pediatr 2012; 19: 1362-8.
6) Sarioglu-Buke A, Corduk N, Atesci F, Karabul M, Koltuksuz
U. A different aspect of corrosive ingestion in children: sociodemographic
characteristics and effect of family functioning.
Int J Pediatr Otorhinolaryngol 2006; 70: 1791-8.
7) Sanchez-Ramirez CA, Larrosa-Haro A, Vasquez-Garibay EM,
Macias-Rosales R. Socio-demographic factors associated with
caustic substance ingestion in children and adolescents. Int J
Pediatr Otorhinolaryngol 2012; 76: 253-6.
8) Rafeey M, Ghojazadeh M, Mehdizadeh A, Hazrati H, Vahedi
L. Intercontinental comparison of caustic ingestion in children.
Korean J Pediatr 2015; 58: 491-500.
9) Temiz A, Oguzkurt P, Ezer SS, Ince E, Hicsonmez A. Longterm
management of corrosive esophageal stricture with balloon
dilation in children. Surg Endosc 2010; 24: 2287-92.
10) Osman M, Russell J, Shukla D, Moghadamfalahi M, Granger
DN. Responses of the murine esophageal micro circulation to
acute exposure to alkali, acid, or hypochlorite. J Pediatr Surg
2008; 43:1672-8.
11) Park KS. Evaluation and management of caustic injuries from ingestion
of acid or alkaline substances. Clin Endosc 2014; 4: 301-7.
12) Uygun I, Arslan MS, Aydogdu B, Okur MH, Otcu S. Fluoroscopicballoon
dilatation for caustic esophageal stricture in children:
an 8-year experience. J Pediatr Surg 2013; 48: 2230-4.
13) Arnold M, Numanoglu A. Caustic ingestion in children-A review.
Semin Pediatr Surg 2017; 26: 95-104.
14) Contini S, Scarpignato C. Caustic injury of the upper gastrointestinal
tract: a comprehensive review. World J Gastroenterol
2013; 19: 3918-30.
15) Temiz A, Oguzkurt P, Ezer SS, Ince E, Hicsonmez A. Predictability
of outcome of caustic ingestion by esophagogastroduodenoscopy
in children.World J Gastroenterol 2012; 18: 1098-103.
16) Riffat F ,Cheng A. Pediatric caustic ingestion: 50 consecutive cases
and a review of the literature. Dis Esophagus 2009; 22: 89-94.
17) Uptodate 26 Feb 2024, https://www.uptodate.com/contents/
caustic-esophageal-injury-in-children
18) Bonavina L, Chirica M, Skrobic O, Kluger Y, Andreollo NA,
Contini S et al. Foregut caustic injuries: results of the world
society of emergency surgery consensus conference.World J
Emerg Surg 2015; 10: 44.
19) Javed A, Pal S, Krishnan EK, Sahni P, Chattopadhyay TK
Surgical management and outcomes of severe gastrointestinal
injuries due to corrosive ingestion.World J Gastrointest Surg
2012; 4: 121-5.
20) Zargar SA, Kochhar R, Mehta S, Mehta SK. The role of fiberoptic
endoscopy in the management of corrosive ingestion
and modified endoscopic classification of burns. Gastrointest
Endosc 1991; 37: 165-9.
21) Cheng HT, Cheng CL, Lin CH, Tang JH, Chu YY, Liu NJ, et al.
Caustic ingestion in adults: the role of endoscopic classification
in predicting outcome. BMC Gastroenterol 2008; 8: 31.
22) Ryu HH, Jeung KW, Lee BK, Uhm JH, Park YH, Shin MH et
al. Caustic injury: can CT grading system enable prediction of
esophageal stricture? Clin Toxicol 2010; 48: 137-42.
23) Lurie Y, Slotky M, Fischer D, Shreter R, Bentur Y. The role of
chest and abdominal computed tomography in assessing the severity
of acute corrosive ingestion. Clin Toxicol 2013; 51: 834-7.
24) Bharath Kumar C, Chowdhury SD, Ghatak SK, Sreekar D, Kurien
RT, David D et al. Immediate and long-term outcome of
corrosive ingestion. Indian J Gastroenterol 2019; 38: 356-61.
25) Baskın D, Urgancı N, Abbasoğlu L, Alkım C,Yalcın M, Karadağ
C et al. A standardized protocol for the acute management of
corrosive ingestion in children.Pediatr Surg Int 2004; 20: 824-8.
26) Anderson KD, Rouse TM, Randolph JG. A controlled trial of
corticosteroids in children with corrosive injury of the esophagus.
N Engl J Med 323:637-40.
27) Ulman I, Mutaf O. A critique of systemic steroids in the management
of caustic esophageal burns in children. Eur J Pediatr
Surg 1998; 8: 71-4.
28) Nunes AC, Romaozinho JM, Pontes JM, Rodrigues V, Ferreira
M, Gomes D et al. Risk factors for stricture development after
caustic ingestion. Hepatogastroenterology 2002; 49: 1563-6.
29) Atabek C, Surer I, Demirbag S, Caliskan B, Ozturk H, Cetinkursun
S, Increasing tendency in caustic esophageal burns
and long-term polytetrafluorethylene stenting in severe cases:
10 years experience. J Pediatr Surg 2007; 42: 636-40.
30) Fernandez FF, Richter A, Freudenberg S, Wendl K, Manegold
BC. Treatment of endoscopic esophageal perforation. Surg Endosc
1999; 13: 962-6.
31) Rentea RM, St Peter SD. Neonatal and pediatric esophageal
perforation. Semin Pediatr Surg 2017; 26: 87-94.
32) Martinez L, Rivas S, Hernández F, Avila LF, Lassaletta L, Murcia
J et al. Aggressive conservative treatment of esophageal
perforations in children. J Pediatr Surg 2003; 38: 685-9.
33) Gander JW, Berdon WE, Cowles RA. Iatrogenic esophageal
perforation in children. Pediatr Surg Int 2009; 25: 395-401.
34) Rentea RM, St Peter SD. Neonatal and pediatric esophageal
perforation. Semin Pediatr Surg 2017; 26: 87-94.
35) Brinster CJ, Singhal S, Lee L, Marshall MB, Kaiser LR, Kucharczuk
JC. Evolving options in the management of esophageal
perforation. Ann Thorac Surg 2004; 77:1475–83.
36) Sanchez TR, Holz GS, Corwin MT, Wood RJ, Wootton-Gorges
SL. Follow-up barium study after a negative water-soluble
contrast examination for suspected esophageal leak: is it necessary?
Emerg Radiol 2015; 22: 539-42.
37) Garey CL, Laituri CA, Kaye AJ, Ostlie DJ, Snyder CL, Holcomb
GW et al. Esophageal perforation in children: a review of
one institution's experience. J Surg Res 2010; 164: 13-7.
38) Mishra B, Singhal S, Aggarwal D, Kumar N, Kumar S. Nonoperative
management of traumatic esophageal perforation leading
to esophagocutaneous fistula in pediatric age group: review
and case report. World J Emerg Surg 2015; 10: 9.
39) Maher MM, Lucey BC, Boland G, Gervais DA, Mueller PR.
The role of interventional radiology in the treatment of mediastinal
collections caused by esophageal anastomotic leaks. Am J
Roentgenol 2002; 178: 649-53.
40) Sasaki T, Culham G, Gandhi SK. Conservative management
of iatrogenic esophageal perforation during neonatal cardiac
surgery. World J Pediatr Congenit Heart Surg 2012; 3: 528-30.
41) Onwuka EA, Saadai P, Boomer LA, Nwomeh BC. Nonoperative
management of esophageal perforations in the newborn. J
Surg Res 2016; 205: 102-7.
42) Orsagh-Yentis D, McAdams RJ, Roberts KJ, McKenzie LB.
Foreign-Body Ingestions of Young Children Treated in US
Emergency Departments: 1995-2015. Pediatrics 2019; 143:
20181988.
43) Mubarak A, Benninga MA, Broekaert I, Dolinsek J, Homan M,
Mas E et al. Diagnosis, management, and prevention of button
battery inges tion in childhood: a european society for paediatric
gastroenterology hepatology and nutrition position paper. J
Pediatr Gastroenterol Nutr 2021; 73 : 129-36.
44) Bekkerman M, Sachdev AH, Andrade J, Twersky Y, Iqbal S.
Endoscopic Management of Foreign Bodies in the Gastrointestinal
Tract: A Review of the Literature. Gastroenterol Res Pract
2016: 8520767.
45) Thomson M, Tringali A, Dumonceau JM, Tavares M, Tabbers
MM, Furlano R et al. Paediatric gastrointestinal endoscopy:
European society for paediatric gastroenterology hepatology
and nutrition and European society of gastrointestinal endoscopy
guidelines. J Pediatr Gastroenterol Nutr 2017; 64: 133-53.
46) Xu G, Chen YC, Chen J, Jia DS, Wu ZB, Li L. Management of oesophageal
foreign bodies in children: a 10-year retrospective analysis
from a tertiary care center. BMCEmerg Med 2022; 22: 166.
47) Raju RS, Raj AJM, Shubha AM. Impacted esophageal foreign
bodies in children. Pediatr Surg Int 2023; 39: 73.
48) Berdan EA, Sato TT Pediatric Airway and Esophageal Foreign
Bodies. Surg Clin North Am 2017; 97: 85-91.
49) Lorie Reilly MSN. Interesting Cases in Pediatric Radiology:
Ingestions, Insertions, and Aspirati ons. J Radiol Nurs 2021;
40: 14-22.
50) Yang W, Milad D, Wolter NE, Propst EJ, Chan Y. Systematic review
of rigid and flexible esophagoscopy for pediatric esophageal
foreign bodies. Int Pediatr Otorhinolaryngol 2020; 139: 110397.






