

Summary
Background: Pulmonary sequestration is defined as nonfunctional lung tissue without a normal tracheobronchial tree that is supplied by an aberrant systemic artery. The awareness of the preoperative diagnosis could be very crucial for the safety of the operation.Materials and Methods: We retrospectively reviewed the records of 16 patients who underwent resection for pulmonary sequestration between 2006-2016. Nine of 16 cases (56%) were female, and the mean age of the patients was 38.5 ± 9.9 years. Fiberoptic bronchoscopy and standard computed thorax tomography were performed for diagnostic work-up in all cases. The patients were divided into 2 groups based on the presence (Group A) or abscence (Group B) of the preoperative diagnosis.
Results: The most common presenting symptoms were cough and expectoration. Preoperative diagnosis of the sequestration was obtained in only 5 patients (31%). Bronchiectasis was the most common cause of false diagnosis, followed by hydatid disease, malignancy, and aspergillosis. Left-sided and intrapulmonary locations were dominant with 12 (75%) and 13 (81%) cases, respectively. Lobectomy was the most common type of surgical resection (75%) and thoracic aorta was the source of aberrant artery in 87% of the patients. Patients in group A were younger. Though intralobar and extralobar types were equally distributed in both groups, all cases in group B had intralobar type. The mean operation time, blood loss, the amount of drainage, and the hospital stay were all insignificantly longer in group B patients. Five of the 6 morbidities occured in group B patients, but the difference was not statistically significant. No mortality occured.
Conclusions: Surgical resection provides definitive management, and is usually reserved for the patients with symptoms. Facilities for a definitive diagnosis should be performed in every case, because, although insignificant, the rate of morbidity is higher in the patients without a definitive diagnosis. Further studies concerning of more patients are required to obtain more comprehensive results.
Introduction
Pulmonary sequestration (PS) is defined as nonfunctional lung tissue without a normal tracheobronchial tree that is supplied by an aberrant systemic artery [1]. It accounts for 0.15-6.4% of all congenital pulmonary malformations [2]. Despite the development of new diagnostic tools like computed tomographic angiography or digital subtraction angiography, the rate of incorrect diagnosis of PS has been reported to be nearly 60% [3]. The correct diagnosis of PS before surgery provides the surgeon to plan the operation, and prevents the unpredictable hemorrhage from the aberrant artery. Failure to diagnose and treat this condition adequately can lead to reccurent pneumonia and even severe hemoptysis. Surgical resection provides definitive management and is usually reserved for patients with symptoms.Methods
We retrospectively reviewed the records of 16 patients who underwent resection for PS at our hospital between 2006-2016. A thorough physical examination was performed, and the signs and symptom of the all patients were recorded. We declare that the study was performed in accordance with the ethical standards laid down in the Helsinki Declaration of 1975, as revised in 1983.All cases had chest X-ray, compturized thorax tomography (CT), routine blood chemistry analysis, and pulmonary function tests.
CT-guided transthoracic aspiration biopsy and indirect hemagglutination tests were performed for solid and cystic lesions, respectively. A positron emission tomography was performed in case of the suspicion for malignancy. Computer tomographic angiography was performed in cases when PS suspicion was quite high. To evaluate the endobronchial anatomy fiberoptic bronchoscopy was performed in all cases. A wedge resection or biopsy from the lesion was performed for for patients. Sputum culture and antibiogram tests directed to appropriate antibiotics.
All surgical procedures, either thoracotomy or video-assisted thoracic surgery (VATS), were performed under single lung ventilation in a posterolateral position. As for thoracotomy, serratus anterior muscle sparing tecnique was preferred. A utility incision and a 10 mm incision for camera port were used for VATS approach. The aberrant artery to the sequestered lobe was dissected carefully, and was either sutured ligated or cut with a stapling device. All cases stayed in the intensive care unit at the day of the operation, and the pain management was achived by patient controlled analgesia.
The patients were divided into 2 groups based on the presence (Group A) or abscence (Group B) of the preoperative diagnosis. Group A consisted of 6 cases with definite diagnosis, and group B without diagnosis(ten cases). Statistical analysis was performed on patients" age, gender, symptom, lesion localization, arterial supply, type of resection, operation time, amount of blood loss, amount of drainage, drainage time, hospital stay, and complications. The data are expressed as mean values ± standard deviation or percentage. Mann Whitney U and Ki square tests were used for comparing the groups. Data were analysed using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, USA) and SPSS version 20.0 software (SPSS,Inc., Chicago, IL, USA).
Results
Nine of 16 (56%) cases were female, and the mean age of the patients was 38.5 ± 9.9 (range 22-56). The most common presenting symptoms were cough and expectoration. Left-sided and intrapulmonary locations were dominant with 12 (75%) and 13 (81%) cases, respectively. Lobectomy was the most common surgical type of resection with 12 patients (75%), and one of them was performed via VATS approach. Except for 2 cases, all sequestered lung tissue were supplied by thoracic aorta. The mean operation time was 148.25 ± 39.86 min, and was longer in the patients undegoing lobectomy. The clinical characteristics of the patients were given in Table 1.Table 1: Clinical characteristics of all patients
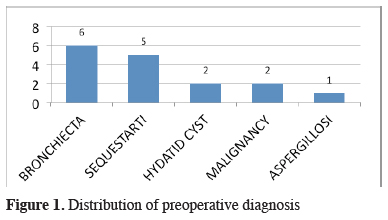
For diagnostic work-up, fiberoptic bronchoscopy and standard computed thorax tomography were performed in all cases. Additional diagnostic tools were listed in Table 2. Preoperative diagnosis of PS was reached in only 5 patients (31%). Bronchiectasis was the most common false diagnosis which was followed by hydatid disease, malignancy, and aspergillosis (Figure 1).
Table 2: Diagnostic tools performed for all patients
 Click Here to Zoom |
Figure 1: Distribution of preoperative diagnosis |
The patients in group A were younger than group B. Though intralobar and extralobar types were disributed equally between the groups, all cases in group B had intralobar type. The mean operation time, blood loss, the amount of drainage, and the hospital stay were all longer in group B, compared to group A. None of the differences between these 2 groups reached significance due to small number of patients in the groups (Table 3).
Six of 16 patients (37.5%) developed complications after the operation. The most severe complication was bronchopleural fistula seen after left lower lobectomy. The patient was treated with tube thoracostomy and fistula was closed on 12th day of the operation. Other complications included hemmorhage requiring thoracotomy, pleural effusion necessitating drainage with pleurocan (8-10F, B. Braun, Melsungen, Germany), and wound infection treated by antibiotics. Five of the 6 morbidity occured in group B patients, but the difference was not statistically significant. No mortality occured.
Discussion
PS is a rare congenital malformation of the lower respiratory tract which is characterized by a solitary non-functioning mass of lung tissue lacking communication with the tracheobronchial tree and arterial blood from the systemic circulation instead of the pulmonary circulation [4]. Though it is first reported by Huber in 1877, Pryce named it as sequestration in 1946 [5]. PSs are divided into two subgroups: intralobar pulmonary sequestration (ILS) and extralobar pulmonary sequestration (ELS). ILS is the more common form and is contained within the normal lung parenchyma, while ELS is separated from the normal lung and has its own visceral pleura [6].The diagnosis of PS was reached in only 5 (31%) of patients. The high rate of bronhiectasis and other infectious diseases like hydatid disease and aspergillosis in our country, and the possibility of malignancy in 2 cases might be the reasons for this relatively low rate of preoperative diagnosis. Moreover one case underwent resection due to massive hemoptysis.
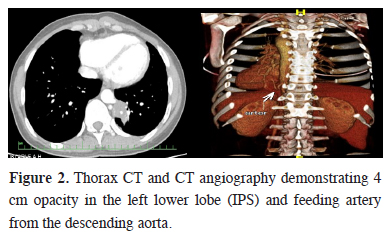
The ability of clearly identifying the aberrant artery to the sequestered lung tissue made the digital subtraction angiography to be the gold standard for the diagnosis of pulmonary sequestration [7]. Nevertheless it is an invasive and radiation-associated procedure that requires hospitalization. In recent years, safer alternatives to digital subtraction angiography, including CT angiography and magnetic resonance imaging (MRI) have proved to be equally effective and less invasive imaging techniques [8]. We performed CT angiography in 5 cases, and identified the aberrant artery to the sequestered lung parenchyma (Figure 2). The clinical findings and thorax CT"s of these cases were strongly suggestive for PS. Though the source of the aberrant artery may be any systemic artery, but in more than 80% of the cases, thoracic aorta is the origin [9,10]. Moreover finding of anomalous systemic arterial supply to basal segments of the lung does not always indicate PS [11]. Sometimes these aberrant arteries develop aneurysmatic dilatation, and cause severe hemoptysis and hemothorax [12-14].
 Click Here to Zoom |
Figure 2: Thorax CT and CT angiography demonstrating 4 cm opacity in the left lower lobe (IPS) and feeding artery from the descending aorta. |
In a recent study, Sun et al. [9] reported that the definitive diagnosis rate of PS before the surgery was 27 out of 72 cases (37.5%). CT angiography was performed in all 27 cases, and 3 of them underwent digital subtraction angiography (DSA) as well. All 5 ELS patients were not diagnosed with PS before surgery in that series. One of the largest series in the literature was reported by Wei et al. [3]. The total number of patients with incorrect diagnosis was 713 of 2625 cases (27%). Unlike our series the main CT appearances of these patients were mass lesions (49%) and cystic lesions (28%). The rate of misdiagnosis of PS as pulmonary cyst and lung cancer were 36.19% and 21.04%, respectively. Contrary to our series (37%) only 15% of patients of that series misdiagnosed as PS. Another recent study from our country reported that only 4 of 11 cases (36%) had the diagnosis of PS, very similar to our results [15]. Contrary to this study, Gezer et al. [1] reported a very low rate (about 10%) of correct diagnosis. They attributed this low rate of correct diagnosis to the study period which was from 1982 to 2006. The authors stressed that CT was not available in the earlier period of the study.
Despite the many studies with low rates of correct diagnosis of PS in the literature, Kestenholz et al. [16] reported 100% of correct diagnosis with 14 cases. The authors stated that the feeding artery was shown with CT scan, and additionally 3 cases had angiography and one had MRI-angiography. Halkic et al. [17] reported one of the highest rate (about 80%) of correct diagnosis. They performed angiography in 20 of 26 patients who had chest X-rays evoking the diagnosis of PS.
In addition to series, there are many case reports of PS in the literature. In those case reports the clinical picture of PS may mimic many diseases, such as hydatid disease, mediastinal mass or even malignacy [18,19]. More interestingly, there are some case reports in which adenocarcinoma was detected in the sequestered lobe [20].
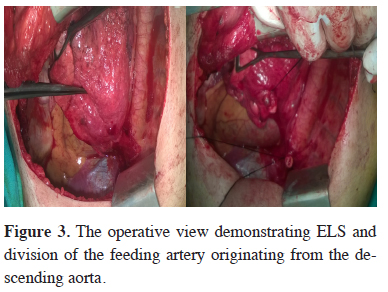
The usual treatment is lobectomy or segmentectomy for ILS, wedge resection and for ELS. The surgical approach extends from open thoracotomy to minimal invasive procedures like VATS or robotic lobectomy. During the resection, the initial step is to identify the aberrant artery which is often localised in the pulmonary ligament (Figure 3). The dissection and finding the aberrant artery is usually challenging because of the adhesions and fibrotic tissue that developed due to previous infections. It is prudent to search for the aberrant artery otherwise inadvertent injury and massive hemorrhage might be unavoidable. Saxxena et al. [21] suggested embolization of the aberrant artery to avoid a catastrophy.
 Click Here to Zoom |
Figure 3: The operative view demonstrating ELS and division of the feeding artery originating from the descending aorta. |
As a conclusion, the diagnosis of sequestration might sometimes be very difficult due to its rareness and inappropriate diagnostic methods. It should be kept in mind that, thorax CT"s of the young adults, especially with symptoms of infectious states, should be carefully checked for a possible aberrant artery to the lung tissue. If neccessary, CT angiography should be performed to define the possible source of the feeding artery. Despite the difficulties such as adhesions and inflammation, which might cause challenges in finding the feeding artery and other hilar structures, surgical resection whether performed by open thoracotomy or minimal invasive approach is quite safe in experienced thoracic centres. Facilities for a definitive diagnosis should be performed in every case, because, although insignificant, the rate of morbidity is higher in patients without a definitive diagnosis. Our study consisted of less number of patients, and was retrospective in nature. These two points are the weakness of this study. Further studies concerning of more patients are required to obtain more comprehensive results.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Reference
1) Gezer S, Taştepe I, Sirmali M, Fındık G, Türüt H, Kaya S, et al. Pulmonary sequestration: a single-institutional series composed of 27 cases. J Thorac Cardiovasc Surg 2007; 133: 955-9.
2) Corbett HJ, Humphrey GM. Pulmonary sequestration. Paediatr Respir Rev 2004; 5: 59-68.
3) Wei Y, Li F. Pulmonary sequestration: a retrospective analysis of 2625 cases in China. Eur J Cardiothorac Surg 2011; 40: e39-e42.
4) Genc O, Gurkok S, Dakak M, Gozubuyuk A, Ozkan M, Caylak H. Pulmonary sequestration and surgical treatment. Asian Cardiovasc Thorac Ann 2006; 14: 3-6.
5) Pryce DM. Lower lobe accessory pulmonary artery with intralobar sequestration of lung: report of 7 cases. J Pathol 1946; 58: 457-67.
6) Savic B, Birtel FJ, Tholen W, Funke HD, Knoche R. Lung sequestration: report of seven cases and review of 540 published cases. Thorax 1979; 34: 96-101.
7) Franco J, Aliaga R, Domingo ML, Plaza P. Diagnosis of pulmonary sequestration by spiral CT angiography. Thorax 1998; 53: 1089-92.
8) Yue SW, Guo H, Zhang YG, Gao JB, Ma XX, Ding PX. The clinical value of computer tomographic angiography for the diagnosis and therapeutic planning of patients with pulmonary sequestration. Eur J Cardiothorac Surg 2013; 43: 946-51.
9) Sun X, Xiao Y. Pulmonary sequestration in adult patients: a retrospective study. Eur J Cardiothorac Surg 2015; 48: 279-82.
10) Osaki T, Kodate M, Takagishi T, Nomi M, Murakami J, Yamamoto H. Unique extralobar sequestration with atypical location and aberrant vessels. Ann Thorac Surg 2010; 90: 1711–2.
11) Gonca C, Hamzayev E, Atasoy C, Enon S. Anomalous systemic arterial supply to normal basal segments of the left lung without sequestration. Eur J Cardiothorac Surg 2015; 47: 578.
12) Shyam S, Sagar A, Sureka J, Jakkani RK. An unusual case of a giant aneurysm of an aberrant systemic artery supplying a pulmonary sequestration. Eur J Cardiothorac Surg 2012; 42: 592.
13) Fabre D, Rohnean A, Fadel E, Dartevelle PG. Giant aneurysmal dilation of an intralobar pulmonary sequestration artery. Eur J Cardiothorac Surg 2009; 36: 413-4.
14) Hofman FN, Pasker HG, Speekenbrink RGH. Hemoptysis and massive hemothorax as presentation of intralobar sequestration. Ann Thorac Surg 2005; 80: 2343-4.
15) Arslan E. Pulmonary sequestration with different clinical and radiological findings. Turk Gogus Kalp Dama 2013; 21: 110-3.
16) Kestenholz PB, Schneiter D, Hillinger S, Lardinois D, Weder W. Thoracoscopic treatment of pulmonary sequestration. Eur J Cardiothorac Surg 2006; 29: 815-8.
17) Halkic N, Cuenoud PF, Corthesy ME, Ksontini R, Boumghar M. Pulmonary sequestration: a review of 26 cases. Eur J Cardiothorac Surg 1998; 14: 127-33.
18) Aydin Y, Ogul H, Altuntas B, Eroglu A. Pulmonary sequestration mimicking a hydatid cyst. Eur J Cardiothorac Surg 2015; 48: 514.
19) Sippel JM, Ravichandran PS, Antonovic R, Holden WE. Extralobar Pulmonary Sequestration Presenting as a Mediastinal Malignancy. Ann Thorac Surg 1997; 63: 1169-71.



