Summary
Background: Small cell lung cancer (SCLC) accounts for 10% of all lung cancers. Due to the fact that it is an aggressive tumor, there is still no consensus on treatment. Our aim in this study was to evaluate prognostic factors affecting survival in early-stage small cell lung cancer.Materials and Methods: Between 2008 and 2017, 21 patients who underwent resection due to earlystage SCLC were evaluated retrospectively in the study. 16 (76.2%) stage I and 5 (23.8%) stage II patients were included in the study. All patients received adjuvant therapy after surgery.
Results: Sixteen (76.2%) male and 5 (23.8) female patients were included in the study. The mean age was 60.19 ± 7.13 years. Patients were followed for a mean of 33 months. There was 16 (76.2%) small cell, and 5 (23.6%) combined small cell carcinoma. The 5-year survival rate was 48.6% (59.6 ± 10.6 months). In 7 patients (33.3%) postoperative morbidity was observed. No statistically significant difference was found between N status and survival. Statistically significant differences were found between tumors smaller than 1 cm and tumors greater than 1 cm in terms of T stage (p = 0.043).
Conclusions: In conclusion, multimodal treatment is very important for survival in early-stage small cell lung cancer. Although our results in our study are aggressive tumors of the SCLC, survival outcomes in the early stages are quite satisfactory. Early stage survival outcomes, especially after stage I tumors, are as successful as non-small cell lung cancers.
Introduction
Lung cancer is still the leading cause of cancer-related deaths. Especially as the population of elderly rise, the incidence of lung cancer increases. The small cell lung cancer (SCLC) accounts for 10% of all lung cancers [1]. For the potential of rapid growth and metastasis of the mediastinal lymph nodes and/or distant metastasis, SCLC’s are regarded as a high-grade malignity. Even though they are chemoradiosensitive tumors; the prognosis is very poor. 5-year survival rates are 5% in the chemotherapy patients [2].Some authors recommend the surgery as a treatment of early-stage SCLC, but the mutual treatment modality is still absent [3]. Our aim in this study is to observe the survival rates of surgically treated SCLC patients and the prognostic factors that affected.
Methods
The ethical committee approval of this study is acquired from Istanbul Education and Research Hospital.Prospectively held data of the surgically treated SCLC patients in our hospital from 1.1.2008 to 1.7.2017 were evaluated retrospectively. Out of 1759 lung cancer patients, 34 were included in the study. 2 of them are lost at follow-up. Seven non-anatomic resection patients and four late-stage patients are excluded from the data. 21 early-stage SCLC patients that underwent a complete resection have evaluated retrospectively. Flowchart of the study has shown in figure 1.
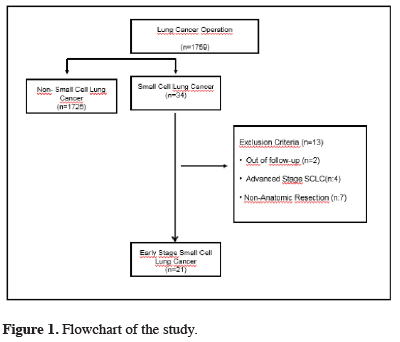 Click Here to Zoom |
Figure 1: Flowchart of the study. |
Preoperative Evaluation
Positron emission tomography (PET-CT), cranial magnetic resonance imaging and computerized thorax tomography (CT) have been used for exploring distant metastasis. Upper and lower abdominal evaluation were performed with CT and PET-CT scans. MRI was requested in the patients who had a lesion in CT. Respiratory function tests and electrocardiography have been used for evaluating preoperative pulmonary and cardiac capacity. Patients with lower than 40% FEV1 results were requested carbon monoxide diffusion test and pulmonary perfusion scintigraphy. All patients were assessed by diagnostic bronchoscopy before surgery. Mediastinal evaluations were performed by endobronchial ultrasonography and /or mediastinoscopy.
Postoperative Follow-up
Demographic data, comorbidities, length of hospitalization, histopathologic features, mortality and 1-2-5 years of survival rates were evaluated in the study.
Comorbidities are examined under three groups. Those groups are; respiratory problems (chronic obstructive pulmonary disease, asthma), cardiac problems (history of myocardial infarction, arrhythmia, history of the coronary stent), endocrinologic problems (diabetes mellitus, goiter) and nephrologic problems (chronic kidney deficiency).
The morbidities that occurred during the hospitalization period or within a month following the operation were included in the study. In this study, we observed empyema, pneumonia, wound infections, arrhythmia and prolonged air leak as morbidities.
Pathologic staging was done according to the 8th edition of the TNM classification system [4]. Patients were followed with chest CT and physical examination every three months for the first two years, every six months between two and five years, and once a year thereafter. All patients were given chemoradiotherapy in the postoperative period.
Surgical Procedure
Following double-lumen selective intubation, patients were placed in the lateral decubitus position for surgery. The patients were operated with posterolateral thoracotomy. After lung resection (lobectomy, pneumonectomy), systematic lymph node dissection was performed. 2, 4, 7, 8, 9, 10 and 11 on the right side and 5, 6, 7, 8, 9, 10, and 11 on the left side lymph node stations were dissected. A single 28-F chest tube was placed in all patients. All patients were extubated in the operation room and transferred to the surgical intensive care unit. They were monitored in the intensive care unit until their general condition stabilized.
Statistical Analysis
SPSS Version 22.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. The chi-squared (χ2) test was used to calculate frequency values as descriptive statistics. A t-test was used to compare the means of independent groups and the Mann-Whitney U was used to compare medians. Survival analysis was conducted according to the Kaplan-Meier method and curves were compared using the log-rank test. The statistical significance level was accepted as p < 0.05 for all analyses.
Results
Out of the 21 patients, 16 patients (76.2%) were male, and five patients (23.8) were female, and the mean age was 60.19 ± 7.13 years (min: 40 max: 71). 15 of the patients (71.4%) were smokers in the preoperative period. Preoperative mean FEV1 value was 77.18 ± 22.2% (min: 42-max: 116). Preoperative comorbidity was present in 12 patients (57.1%). Demographic characteristics of the patients are listed in table 1.Table 1: Demographic characteristics of patients with early stage SCLC
Nine patients (40.9%) underwent lung resection on the right side and 12 patients on the left side (59.1%). Most common resection was left upper lobectomy (%31.8). Pneumonectomy was performed in 2 patients with the central tumor. Preoperative tumors of these patients were reported as non-small cell lung cancer. Postoperative pathology was reported as combined small cell carcinoma. Mean tumor diameter was 2.02 ± 1.80 cm. 16 (76.2%) of the patients, were small cell carcinoma and 5 (23.8%) patients were combined small-large cell carcinoma. Intraoperative mean 4.7 ± 1.64 lymph node stations were sampled. The number of lymph nodes removed was 12.19 (min: 4 - max: 34). The surgical and histopathological features of the patients mentioned in table 2.
Table 2: Surgical and histopathological characteristics of patients.
There was no mortality in the following 30 days of surgery. Postoperative morbidity was observed in seven patients (33.3%). 13 morbidity was observed in seven patients. Seven patients with prolonged air leak (33.3%), one patient had (4.8%) pneumonia, three patients developed (14.3%) atrial fibrillation, one patient had (4.8%) wound infection and one (4.8%) patient with empyema. Air leakage was spontaneously regressed in 5 patients, and two patients needed blood pleurodesis. Medical cardioversion treatment was applied to patients with atrial fibrillation. Antibiotic therapy was performed according to the culture results to patients with empyema. There was no statistically significant relationship between morbidities with co-morbidity, age, sex, side, and operation pattern (p > 0.05).
Mean follow-up time for patients is 33 months. 5-year survival rate was calculated as 48.6% (59.6 ± 10.6 months). No statistically significant difference was found between N status and survival (p = 0.416). No difference was found in isolated SCLC when group analysis was performed (p = 0.733). When compared in the T stage, there was no significant difference in survival rates (p = 0.240). The analysis revealed a statistically significant difference (p = 0.043) in the group analysis between 1 cm and below tumors and above than 1 cm ones. The 5-year survival rate for 1 cm and below tumors was found 100%, while the survival rates of the patients with tumors above than 1 cm was 27.1%. Table 3 shows prognostic factors affecting survival in early stage SCLC.
Table 3: Factors affecting survival in early stage SCLC.
Postoperative metastasis was detected in 10 patients (52.4%). 4 patients (19%) had lung, 4 patients (19%) had cranial, and 2 patients (9.5%) had vertebral metastasis. The disease-free survival was 28 months, the 2-year DFS 60% 5-year disease-free survival rate was 0%. 5-year disease-free survival was 41.9% in patients undergoing prophylactic cranial radiotherapy (PCI) and 0% in non-patients (p = 0.041). Kaplan Meier survival graph was shown in figure 2. There was no statistically significant difference in DFS in terms of resection type, stage, N status, sex, and histopathology.
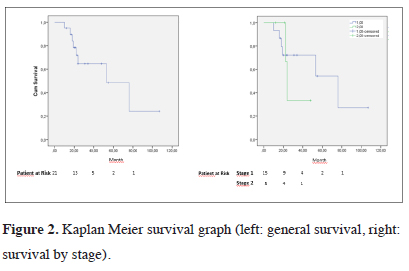 Click Here to Zoom |
Figure 2: Kaplan Meier survival graph (left: general survival, right: survival by stage). |
Discussion
The prognosis of SCLC is very poor because they are aggressive tumors. Survival rates of patients treated with chemoradiotherapy, even when they are detected at an early stage, is only 15% [5-7]. In today’s standards, place of surgical treatment of SCLC is not clear, only to be used in a part of bi-trimodal treatments. The main reasons for this are; the difficulty of diagnosing SCLC at early stages while they still have resection potential [5], the insufficiency of standalone surgical treatment. (especially in the 1970’s British Medical Research study, the outcomes of surgical treatment alone were quite bad) [8]. Therefore, surgical treatment is recognized as a component of multimodal treatment [9-11].In a retrospective study of 39 patients with Bischof et al [12], the 5-year survival rate was 49%. In a 67 patient study of Badzio et al [13] showed that the five-year survival of patients who were performed surgery was 27%, while chemoradiotherapy-only patients’ survival rate was 4%. Japan Clinical Oncology Group found that 5-year survival was 67% in stage 1A and 73% in stage 1B in SCLC. They have performed prophylactic cranial radiotherapy to reduce the risk of cranial metastasis. Local recurrence was observed in 10% of patients, and cranial metastasis was observed in 15% of patients in that study. In 2009, 13,290 SCLC cases were identified from the IASLC database. 349 of which had performed R0 resection. Five-year survival rates for them were 48%, 39% and 15% in stage I, II and III respectively [14]. It was also found that the survival outcomes were inversely proportional to T and N. In 2018, Paximadis et al [3] compared the surgery with SBRT and external beam radiation therapy (EBRT) in Stage 1 SCLC. Overall survival (OS) was more successful than EBRT and SBRT in patients who underwent surgery (p < 0.001). Additional use of chemotherapy with any treatment modality resulted in improved OS (p < .001). Che et al [15] reported better survival rates in patients who underwent surgery in stage I and II (p < 0.001). Survival was observed in patients who did not undergo surgery for two months, and the survival of patients who were undergoing lobectomy was 34 months In our study, the 5-year survival rate in stage I tumors were 54.2% in patients with SCLC, and the 2-year survival rate was 33%. Comparison between the studies is shown in table 4.
Table 4: Assessment of survival in the presence of literature.
It is recommended to perform detailed mediastinal and distant lymph node metastasis assessment to all patients that planning surgery for primary SCLC [14,28]. In a study done by using Surveillance Epidemiology and End Results (SEER) database, Yu et al [29] Found that the survival outcomes in patients with mediastinal lymph node involvement were worse than those without mediastinum involvement. They also found that the stage I patients that underwent lobectomy had a 50% survival rate [29]. Schreiber et al [23] have found similar survival outcomes in a study of 861 SCLC patients, done with the SEER database (5-year survival 53%). However, N1 and N0 have been reported to be beneficial to patients. In this study, we found N0 patients’ 5-year survival rate of 54.2% and N1 patients’ 2-year survival rate of 33%. No statistically significant results were found due to the low number of patients in the study. Similar to the literature, T1a tumors have better survival outcomes than the other stages in terms of T stage.
In our study, DFS was 60% in 2 years and 0% in 5 years. Prognostic factor affecting especially DFS is prophylactic cranial radiotherapy. Bang et al reported that there was a statistically significant difference in overall survival in patients receiving PCI (HR 0.55; 95% CI: 0.39-0.77; P = .0005) and time to brain metastasis (HR 0.40; 95% CI: 0.23-0.66; P = .0004). Although our results were found to be similar to the literature, no subgroup analysis could be performed due to the small number of patients in the study.
The main limitations of this study are the low number of patients, being retrospective and lack of a control group of patients who only treated with chemoradiotherapy. However, retrospective comparisons will not be feasible as the performance status of patients receiving surgery is better than patients receiving chemoradiotherapy. Furthermore, there aren’t any studies in the literature that compared surgical treatment with chemoradiotherapy treatment as of today.
In conclusion, multimodal therapy is very important for survival in early stage SCLC. Even though SCLC’s are very aggressive tumors, the results of our study show that survival outcomes in the early stages are quite satisfactory. Especially, in stage 1 tumors short-term survival rates are shown as successful as non-small cell lung cancers. Therefore surgical treatment should be considered before definitive chemoradiotherapy in early stage SCLC. However, further prospective studies are needed for the evaluation of surgical treatment versus medical therapy.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Reference
1) Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 39-51.
2) Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59: 225–49.
3) Imamura F, Ueno K, Kusunoki Y, Uchida J, Yoshimura M, Koizumi M, et al. High-dose-rate brachytherapy for small-sized peripherally located lung cancer. Strahlentherapie Und Onkol 2006; 182: 703.
4) Cooper S, Spiro SG. Small cell lung cancer: treatment review. Respirology 2006; 11: 241–8.
5) Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus: ten-year follow-up. Lancet 1973; 302: 63–5.
6) Waddell TK, Shepherd FA. Should aggressive surgery ever be part of the management of small cell lung cancer? Thorac Surg Clin 2004; 14: 271–81.
7) Anraku M, Waddell TK. Surgery for small-cell lung cancer. Semin. Thorac. Cardiovasc. Surg., vol. 18, Elsevier; 2006, p. 211–6.
8) De Antonio DG, Alfageme F, Gamez P, Cordoba M, Varela A. 63. Bronchogenic Carcinoma Cooperative Group of the Spanish Society of Pneumology and Thoracici Surgery (GCCB-S). Results of surgery in small cell carcinoma of the lung. Lung Cancer 2006; 52: 299–304.
9) Bischof M, Debus J, Herfarth K, Muley T, Kappes J, Storz K, et al. Surgery and chemotherapy for small cell lung cancer in stages I–II with or without radiotherapy. Strahlentherapie Und Onkol 2007; 183: 679–84.
10) Badzio A, Kurowski K, Karnicka-Mlodkowska H, Jassem J. A retrospective comparative study of surgery followed by chemotherapy vs. non-surgical management in limited-disease small cell lung cancer. Eur J Cardio-Thoracic Surg 2004; 26: 183–8.
11) Vallieres E, Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009; 4: 1049–59.
12) Fujimori K, Yokoyama A, Kurita Y, Terashima M. A pilot phase 2 study of surgical treatment after induction chemotherapy for resectable stage I to IIIA small cell lung cancer. Chest 1997; 111: 1089–93.
13) Rea F, Callegaro D, Favaretto A, Loy M, Zuin A, Sartori F. Results of surgery and chemotherapy in small cell lung cancer. Lung Cancer 1999; 25: S23.
14) Rostad H, Strand T-E, Naalsund A, Norstein J. Resected synchronous primary malignant lung tumors: a population-based study. Ann Thorac Surg 2008; 85: 204–9.
15) Brock M V, Hooker CM, Syphard JE, Westra W, Xu L, Alberg AJ, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J Thorac Cardiovasc Surg 2005; 129: 64–72.
16) Tsuchiya R, Suzuki K, Ichinose Y, Watanabe Y, Yasumitsu T, Ishizuka N, et al. Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I-IIIa small cell lung cancer: the Japan Clinical Oncology Lung Cancer Study Group Trial (JCOG9101). J Thorac Cardiovasc Surg 2005; 129: 977–83.
17) Granetzny A, Boseila A, Wagner W, Krukemeyer G, Vogt U, Hecker E, et al. Surgery in the tri-modality treatment of small cell lung cancer. Stage-dependent survival. Eur J Cardio-Thoracic Surg 2006; 30: 212–6.
18) Lim E, Belcher E, Yap YK, Nicholson AG, Goldstraw P. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol 2008; 3: 1267–71.
19) Shepherd FA, Ginsberg RJ, Patterson GA, Evans WK, Feld R. A prospective study of adjuvant surgical resection after chemotherapy for limited small cell lung cancer. A University of Toronto Lung Oncology Group study. J Thorac Cardiovasc Surg 1989; 97: 177–86.






