2Department of Thoracic Surgery, Bolu Abant Izzet Baysal University, Bolu, Turkey DOI : 10.26663/cts.2019.00031
Summary
In this review, the causes of pleural effusion were discussed. Pleural reactions and tumors were explained in the tables. Subsequently, radiographic findings and their role of guidance in the interventional procedures were briefly mentioned. Surgical diagnosis and treatment methods were explained. Malignant mesothelioma was discussed under a separate title. In the end, pleurodesis, a commonly used method in patients with malignant pleural effusion, was discussed.Introduction
Introduction and EtiologyThe characteristic of malignant pleural effusion (MPE) is the presence of cancerous cells in pleural fluid or pleural tissue obtained at percutaneous needle biopsy or thoracoscopy, thoracotomy, or autopsy. Malignant pleural effusion is a highly morbid disease [1,2]. 80% of MPEs are caused by lung, breast, over, gastrointestinal cancers, and lymphomas. Primary malignancy may not be detected in about up to 10% of MPEs. 95% is unilateral. Lung cancer is the leading causes of MPE [1,2].
Almost all tumor types may cause a malignant pleural effusion. The prognosis is based on tolerability of the patients to systemic treatment; however, the presence of malignant effusion is not associated with pathologic diagnosis or indication for poor prognosis. There is no specific guideline for the treatment of malignant pleural effusion and evidence to dope out the survival of these patients [3]. The development of pleural effusion in a cancer patient does not indicate that this fluid is malignant. This may be due to atelectasis and pneumonia, due to bronchial constriction, or as a side effect of severe malnutrition, cardiac failure, chemotherapy drugs, or radiotherapy.
Rare pleural malignant tumors may also cause malign pleural effusion besides malignant mesothelioma and other common pleural tumors (Table 3).
Table 1: List of the conditions that lead to pleural effusion via causing a pleural reaction [4].
Table 2: World Health Organization classification of pleural cancers [5].
Table 3: Malign tumors of pleura other than mesothelioma [6].
Radiological Findings
Posterior-Anterior Lung Graph;
The mediastinum is shifted to the rightward. 200-300 cc fluid is sufficient for blunting the costodiaphragmatic recess. Lateral chest graph, lateral decubitus graph or USG are crucial at the differential diagnosis of fluid, atelectasis, pneumonia, and pleural thickening. On PA roentgenogram the affected hemithorax shows completely homogeneous density.
When the collection was too much, the entire hemithorax is covered with homogeneous density and mediastinum was pushed to the opposite side. Chest x-ray appearance in intrapulmonary pleurisy mimics a diaphragm elevation [7].
Thorax ultrasonography
Using the B-Mode technique, the echo waves reflected from intra-thoracic structures are evaluated with the guidance of intercostal windows. The application process is easy, and sonographer can get results after the evaluation (Figure 2). Initial indications: Ultrasonography is essential in the diagnosis of localized fluids. It is critical to distinguish effusion locations to see pleural fluid without pleural thickening, before pleural biopsy or thoracic tube placement. It is used to determine the amount and localization of pleural effusion, to separate the pleural thickening from minimal pleural effusion and to obtain a parietal pleura biopsy. The preferences to use USG rather than computerized tomography in the pleural fluid could be summarised as follows: USG is easy and quick to handle, the patient does not take the radiation, it is cheap, and USG can be used as a guidance to locate the thoracentesis. USG detects pleural fluid septations with higher sensitivity than CT [7].
 Click Here to Zoom |
Figure 1: Chest x-rays showing pleural effusion. |
 Click Here to Zoom |
Figure 2: Thorax ultrasonography showing pleural effusion. |
Computerized Thorax Tomography
CT is important for the diagnosis of local fluids. Since computerized tomography (CT) has become widespread and accessible, it is generally the first choice after the chest x-ray. Computerized tomography is crucial in every undiagnosed exudate fluid (Figure 3). In addition to exudate fluid, it is more useful at recognition and identification of the parenchymal pathology, pleural mass, and nodules [7].
 Click Here to Zoom |
Figure 3: Computerized thorax tomography showing pleural effusion and thickening. |
Surgical Diagnosis and Treatment Methods
After the physical examination of the patient with pleural effusion, the above-mentioned radiological examinations should be performed. If the patient has exposure to asbestos and there is a radiological suspicion of mesothelioma, these patients should also be assessed as described in the section of the malignant pleural mesothelioma. Transudate/exudate fluid differentiation should be made from the fluid collected via thoracentesis. Also, cytological, other biochemical, and microbiological examinations should be done. In doubt of malignancy, the patient should undergo bronchoscopy and interventional diagnostic procedures, according to results of tomography [8]. The methods are as shown in table 4.
Table 4: Surgical diagnosis and treatment methods.
1. Thoracentesis
The first procedure to perform in a patient with malignant pleural effusion is thoracentesis. Thoracentesis can be useful for both diagnosis and elimination of the most common complaint, the dyspnea. Also, lung expansion capacity can be evaluated during the procedure. If the patient’s life expectancy is short (especially less than a month), then the treatment can be continued with evacuation thoracentesis. The ideal treatment choice should be tube thoracostomy and pleurodesis. In many patients, fluid is drained via repeated thoracentesis to provide symptomatic relief. But there are some disadvantages of this implementation. First, the fluid is recollected shortly after thoracentesis. In addition, the risk of infection is high, and inoculations may develop in the pleural fluid (Figure 4).
 Click Here to Zoom |
Figure 4: Thoracentesis procedure. |
For thoracentesis, preferred application location is intercostal space under the fluid level. In order not to damage the intercostal vein and nerve pack, the punch is applied from the upper part of the costa. Major complications are cough, pneumothorax, chest pain, infection at the pleural cavity, hemothorax, vasovagal reflex stimulation (bradycardia, hypotension), and rarely diaphragm, liver or splenic injury [9].
2. Closed pleural biopsy
This technique can be applied with Abrams, Cope, Vim-Silverman needles (Figure 5). This procedure, like thoracentesis, is performed via inserting the notched end of the pleural biopsy needle into the pleura and by performing a biopsy with the drill inside. Diagnostic value of closed tubal pleura biopsy is 50-80%. If pleural fluid cytology is negative for malignant pleural fluid, a closed pleural needle biopsy may be performed, but in this case, the diagnosis rate is decreasing. A thoracoscopic biopsy is more commonly used in exudative pleural fluids that cannot be diagnosed instead of pleural needle biopsy. Thoracentesis and closed pleural biopsy can be done in outpatient clinics, but there are complication risks such as hemoptysis and hemothorax. Closed pleural biopsies are less preferred because of their low diagnostic yield and relatively high complications. This method can be preferable for the patients with tuberculous pleurisy [10].
 Click Here to Zoom |
Figure 5: Abrahams needle set. |
3. Biopsy with imaging modalities
In the context of imaging modalities, especially with the guidance of thoracic tomography and thorax ultrasonography, a tru-cut biopsy is the preferred method with high diagnostic and low complication rate. MR and PET/CT guidance has begun to become the current method for biopsies [10].
4. Thoracoscopy with local anesthesia
Pleuroscopy method, thoracoscopy with local anesthesia, can be useful to take biopsies from pathological areas. Cryobiopsy, as a novel sampling method, which freezes the samples during flexible or rigid thoracoscopy, can be a new option in the undiagnosed effusions [10,11].
5. VATS
Video-Assisted Thoracoscopy (VATS) is frequently used due to its development in today’s state-of-art technology, which is the procedure performed under the preference sedoanalgesia or general anesthesia according to the patient’s choice (Figure 6).
 Click Here to Zoom |
Figure 6: VATS procedure. |
With VATS, the hemithorax can be visualized in the best way, and the biopsies can be obtained with the highest diagnostic value. In selected eligible patients, partial pleurectomy can also be performed to provide lung expansions. The entire pleural field exploration is the procedure which allows complete pleurodesis, including complete confirmation of the lung expansion and application of talc with visual-assistance for the removal of the entire fluid. Thoracoscopy is a safe procedure and mortality is reported to be 0.1% in 1350 cases [3,12].
6. Open Pleural Biopsy
An open pleural biopsy may be performed in patients with pleural adhesions that are not appropriate for VATS. This procedure can also be performed under sedation, and local anesthesia where the general condition of the patient is not good and general anesthesia cannot be obtained [3].
7. Major Resections
Surgical options are VATS pleurodesis, VATS partial pleurectomy, pleurectomy / decortication, extended pleurectomy/decortication, and extrapleural pneumonectomy. In some studies, mortality and survival advantage has been shown to be comparable to the others in Extended Pleurectomy / Decortication surgery. However, there are prospective, randomized controlled trials studies which show the comparison between these surgical options [3]. In the past, these procedures were performed with open thoracotomy and also with VATS since the early 1990s. Pleurectomy is performed in two conditions. First one is the thoracotomy for pleural effusion that is not diagnosed. If malignancy is detected, immediate parietal pleurectomy is performed to prevent fluid accumulation. The second condition is persisting pleural effusion, and sclerosing agent cannot be applied due to the restricted lung. Parietal pleurectomy is performed with decortication. Technically, dissection is advanced according to the location of the parietal pleural tumor. After reaching to the mediastinal surface, the primary aim should be not to damage the phrenic nerve, the recurrent laryngeal nerve, the sympathetic nerves or the stellate ganglia or vascular structures (Figure 7). Diagnosticians should care not to remove the diaphragmatic connection parts of the chest wall in the costophrenic sinus, because the application is unnecessary and often impossible, and decortication should be added only if the visceral lung pleura is not expansive depending on the fibrin texture. Another major resection is extrapleural pneumonectomy. Additional intra-operative or post-operative treatments should be performed when major resection is planned. These are hyperthermic intraoperative chemotherapy and photodynamic therapy. In addition, radiotherapy could be added [13,14].
 Click Here to Zoom |
Figure 7: Application of parietal pleurectomy with VATS. |
8. Permanent Pleural Catheter / Pleuroperitoneal Shunt
Persistent pleural catheters can be applied in cases where the pleural effusion cannot be drained, and the lung cannot be expanded. And patients may be monitored in the outpatient clinic [15]. Permanent pleural catheters used more frequently in recent years and recommended for patients with frequent, recurrent, and inoperable pleurodesis [16]. The pleuroperitoneal shunt is another option when patients are symptomatic, pleurodesis cannot be performed, and lung is not expansive. It is preferred in cases where the respiratory parameters are limited, the general condition is not very good, and the pleural function is not considered. It allows the lung to ventilate, prevents mucous retention, atelectasis, and pneumonia. Tumor cells have a risk of spreading to the peritoneal cavity and shunt failure due to catheter blockage may happen [17].
Malignant Mesothelioma
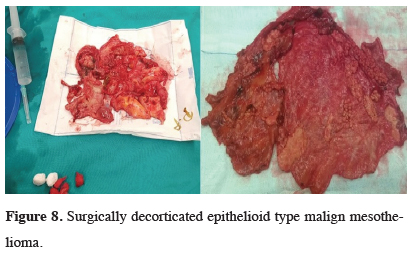
A separate section is needed to approach the patient with pleural effusion and doubt of malignant mesothelioma. Pleural effusion is usually the first clinical manifestation of malignant mesothelioma. The guidelines prepared by ERS and ESTS for diagnosis and treatment of malignant pleural mesothelioma include the following suggestions. Cytological examination in pleural effusions is the first choice in diagnosis. However, the cytological examination is not preferable for patients with doubt of the MPM, since the misdiagnosis rate is too high (Grade IB). When cytologically suspicious, this condition should be confirmed with tissue biopsy (Grade IB). Closed pleural biopsies (Abrams or Castelain’s needles) have a higher error rate, as in cytologic diagnosis. Thoracoscopy is recommended as a diagnostic method (Grade IA). With thoracoscopy, the entire pleura could be observed, and deep biopsies could be obtained from both normal and pathological areas (Figure 8). Fat and muscle invasions can also be detected during the procedure [18].
 Click Here to Zoom |
Figure 8: Surgically decorticated epithelioid type malign mesothelioma. |
Pleurodesis
Side effects, cost-effectiveness, and ease of administration should be considered in selecting the preferred agent for pleurodesis. The success rate of talc pleurodesis is high. Large particulate talc pleurodesis should be preferred to avoid possible ARDS-like complications.
Before the pleurodesis procedure, a diluted local anesthetic is delivered via tube thoracostomy. After that, a sclerosing agent dissolved or suspended in 50-100 ml of saline should be administered to the pleural cavity. With this method, the irritation pain of the patient during the procedure can be alleviated. After the procedure, the tube is clamped and left in this position for 3-4 hours. The tube can be aspirated so that it does not exceed 20 cm H2O pressure.
If the patient develops severe pain or shortness of breath after the procedure, the tube should be opened immediately, and the talc should be drained. If the drainage falls below 50-100 ml, the tube thoracostomy should be terminated [19].
Pleurodesis can also be done with VATS technique. During VATS for diagnostic purposes, in patients suspicious for a pleural tumor or metastasis, frozen examination can be done. For those who will not undergo additional surgery, the fluid may be drained entirely, and talc may be applied after the adhesions have been removed [20].
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Reference
1) Paul S, Zellos L. Malign plevral efüzyonun cerrahi dışı tedavisi. In Sugarbaker DJ, Bueno R, Krasna MJ, Mentzer SJ, Zellos L (eds). Erişkin Göğüs Cerrahisi. Çeviri Editörü: Mustafa Yüksel, Nobel Tıp Kitabevleri, İstanbul 2011; 849-54.
2) Panadero FR. Effusions from malignancy. In Light RW, Lee YCG (eds). Textbook of Pleural Disease, 2nd ed. Lipincott Williams & Wilkins, 2008; 323-40.
3) Perikleous P, Waller DA. Video assisted thoracoscopic and open chest surgery in diagnosis and treatment of malignant pleural diseases. J Vis Surg 2017; 3: 85.
4) McNamee J. Plevranın Benign Tumorleri. In Sugarbaker DJ, Bueno R, Krasna MJ, Mentzer SJ, Zellos L (eds). Erişkin Goğus Cerrahisi. Ceviri Editoru: Mustafa Yuksel, Nobel Tıp Kitabevleri, İstanbul 2011; 1068-76.
5) Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC. Pathology and Genetics of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press, 2004; 126-44.
6) Shields TW, Yeldani AV. Uncommon Tumors of the Pleura. In: Shields TW, Lo Cicero III J, Reed CE, Feins RH, General Thoracic Surgery, seventh ed, Vol I, Lippincott Williams & Wilkins, Philadelphia 2009; 869-73.
7) Maskell N, Butland NR. BTS guidelines for the investigation of a unilateral pleural effusion in adults. Thorax 2003; 58: ii8.
8) Scherpereel A. Pleural and Chest Wall Tumours. ERS Handbook, Respiratory Medicine, First Edition 2010, Page: 392-9.
9) Li Xiao, Ferguson MK. Optimal management of symptomatic malignant effusion. In MK Ferguson (ed.). Difficult Decisions in Thoracic Surgery. An Evidence-based approach. Springer-Verlag London. 2014. p. 635-45.
10) Bibby AC, Maskell NA. Pleural biopsies in undiagnosed pleural effusions; Abrams vs image-guided vs thoracoscopic biopsies. Curr Opin Pulm Med 2016; 22: 392-8.
11) Thomas R, Karunarathne S, Jennings B, Morey S, Chai SM, Lee YC, Phillips MJ. Pleuroscopic cryoprobe biopsies of the pleura: a feasibility and safety study. Respirology 2015; 20: 327-32.
12) Keller, Steven M. Current and future therapy for malignant pleural effusion. Chest 1993; 103: 63S.
13) Fry WA, Khandekar JD. Parietal pleurectomy for malignant pleural effusion. Ann Surg Oncol 1993; 2: 160-4.
14) Friedberg JS. Photodynamic therapy in the management of malignant pleural effusions. In: In Sugarbaker DJ, Bueno R, Krasna MJ, Mentzer SJ, Zellos L, eds. Adult Chest Surgery. McGraw-Hill; 2009: 891-9.
15) Tremblay A, Michaud G. Single-center experience with 250 tunneled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129: 362-8.
16) Suzuki K, Servais EL, Rizk NP Solomon SB, Sima CS, Park BJ et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunelled pleural catheters. J Thorac Oncol 2011; 6: 762-7.
17) Al-Kattan KM, Kaplan DK, Goldstraw P. The nonfunctioning pleuroperitoneal shunt: revise or replace? Thorac Cardiovasc Surg 1994; 42: 310-2.
18) Scherpereel A, Astoul P, Baas P, Berghmans T, Clayson H, de Vuyst P et al. ERS/ESTS Task Force Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010; 35: 479–95.






