

2Department of Thoracic Surgery, Yedikule Thoracic Surgery Training and Research Hospital, Istanbul, Turkey DOI : 10.26663/cts.2019.0003
Summary
Background: The role of fluids in the etiology of postpneumonectomy pulmonary edema (PPE) is controversial. The aim of this experimental study was to assess the effect of crystalloid or colloid fluids and normal or high volume on the etiology of PPE.Materials and Methods: 20 New Zealand rabbits were used and divided into 4 groups. Pneumonectomy had performed firstly in each subject, then for 3 hours, normal and high volume of crystalloid and colloid fluids were administered. The first group were administered 3 ml/kg/h crystalloid (0.9% NaCl), the second group received 10 ml/kg/h of the same crystalloid, the third group were administered 3 ml/ kg/h colloid (6% HES/0.7) and the fourth group received 10 ml/kg/h of the same colloid infusion. Pneumonectomy and remaining lung materials were weighed when wet and dry and pathologic investigation was performed with a light microscopy.
Results: There was no significant difference between the preoperative and postoperative wet/dry ratio (W/DR) of pneumonectomy and remaining lung material in all groups administered crystalloid and colloid (1st Group Z = 0.707; p = 0.480, 2nd Group Z = 0.577; p = 0.564, 3rd Group Z = 0.577; p = 0.564, 4th Group Z = 0.677; p = 0.498). There was no significant difference identified between pneumonectomy material W/DR for all crystalloid and colloid groups (x2 = 2.787; p = 0.426) and again between the remaining lung material W/DR (x2 = 2.858; p = 0.414). The histopathologic assessments of all groups were similar.
Conclusion: Administration of crystalloid or colloid fluids at normal or high volume was assessed not be responsible alone for the PPE etiology in subjects with normal cardiac function. Histopathologic findings were similar in all groups.
Introduction
Pulmonary edema (PE) was first described by Gibbon and Gibbon in 1942 in their study of cats [1]. PE may form due to any factor causing pulmonary interstitial fluid pressure to increase from negative values to positive values. The most common cause of PE not due to cardiac sources is disruption or destruction of pulmonary capillary membrane [2]. Factors causing increases in pulmonary capillary hydrostatic pressure or damaging alveolocapillary membrane may cause PE. With pneumonectomy, vital capacities fall by 35-40%. Fluid accumulation in the remaining lung fills the interstitial field and alveoli with fluid causing surfactant inhibition. Thus, the ventilated pulmonary field reduces inducing serious hypoxia [3]. Fluid accumulation in the remaining lung leads to high rates of 4-5% postoperative mortality [4].Non-cardiac sourced PE following pneumonectomy was first described by Zeldin et al. When first described the most important cause was excessive fluid loading [5]. Many experimental studies have been performed about the etiology of postpneumonectomy pulmonary edema (PPE) and other factors have been identified. Causes such as increased pulmonary capillary hydrostatic pressure, increased permeability, hormonal factors, mediastinal lymph node dissection and hyperinflation with ventilator treatment are reported [6]. Additionally, there are publications showing fluids do not play a role [7,8]. Thus, other mechanisms have been revealed while the role of fluids in PPE is still controversial.
In this study, our aim was to investigate whether non-cardiogenic PE is induced in the remaining lung in a rabbit model administered crystalloid and colloid fluids and different amounts of fluid after pneumonectomy.
Methods
A total of 20 New Zealand rabbits with a mean weight of 1.831 g (range, 1.400 to 2.900 g) were used in this study. All animals treated and cared for in accordance with the TU, Institutes of Health Guide for the care and Use of Laboratory Animals and the research was conducted in accordance with the 1964 Helsinki Declaration. The subjects were divided into four groups including five in each group. The first group was given 3 mL/kg/h of crystalloid (isotonic 0.9% NaClR, Fresenius Kabi AG, Bad Homburg, Germany) the second group was given 10 mL/kg/h of crystalloid (isotonic 0.9% NaClR, Fresenius Kabi AG, Bad Homburg, Germany); the third group was given 3 mL/kg/h of colloid (6% HESR 450/0.7, Fresenius Kabi AG, Bad Homburg, Germany), and the fourth group was given 10 mL/kg/h of colloid (6% HESR 450/0.7, Fresenius Kabi AG, Bad Homburg, Germany). All groups received infusion for three h. The subjects were anesthetized with 10 mL/kg of ketamine (Ketalar 50 mg/mL, 10 mL flask, Phizer, Kent,UK) and 0.3 mL/kg xylazine hydrochloride (Rompun 50 mL flask, Bayer, Leverkusen, Germany) and placed in a supine position. The jugular veins of the subjects were dissected using a vertical incision on the neck. They were catheterized using 24F branule. 1 mg of ethylenediaminetetraacetic acid (EDTA) in 0.9 mL and 0.1 mL (TrasylolR 10,000 U/mL, 50 ml flask, Bayer, Leverkusen, Germany) aprotinin-containing plastic tubes were prepared. 1 mL of blood was drained into these previously prepared and cooled. These blood samples were centrifuged at 2,000 g, -4°C for 30 min. Blood plasma was separated and kept at -30°C. After the blood was drawn, 3 mL/kg/h or 10 mL/kg/h crystalloid or colloid fluid were administered via a perfusion pump (Braun Perfusor, Melsungen, Germany).Meanwhile, the trachea was reached and incised by expanding the incision to the neck. The subjects were intubated with a 3.0 intubation tube and were ventilated with continuous positive airway pressure with O2 (tidal volume: 4 mL/kg; respiratory rate 45-60/min; positive airway pressure: 1-2, 30% O2, Vokar- Sav: 0301). The rabbits were placed in lateral decubitus according to the side to which the pneumonectomy was to be applied. Posterolateral thoracotomy was applied to all subjects. The pulmonary inferior ligament was divided, then, isolated and the hilus was tied and cut en bloc with 2/0 silk, and pneumonectomy was performed.
In the first group, left pneumonectomy and right pneumonectomy were performed in three and two subjects, respectively. In the second group, left pneumonectomy and right pneumonectomy were performed in two and three subjects, respectively. In the third group, left pneumonectomy and right pneumonectomy were performed in three and two subjects, respectively. In the fourth group, left pneumonectomy and right pneumonectomy were performed in three and two subjects, respectively. The blood samples from the contralateral jugular vein were collected into plastic tubes, as described. The pneumonectomy procedure was repeated for the contralateral lung and all subjects were sacrificed.
For pathology of pneumonectomy and remaining lung material, a 2x2 mm biopsy was taken from the lower lobe posterobasal segment and immediately weighed with a sensitive scale when wet to prevent losses due to evaporation. Later pneumonectomy and remaining lung material were dried for 24 hours in an 80 °C oven and weighed again with the same scale, similar to the literature [8,9]. Both materials had W/DR and percentages identified. Material taken for pathology was left in formaldehyde and then assessed under a light microscope.
Statistical Analysis
The data were collected and analyzed using Excel software (Microsoft Corp, Seattle, WA). Descriptive statistics were used to report the means and standard deviations of the continuous variables and number and percent of categoric variables. The Kruskal-Wallis test was used to compare mean weight of the subjects. The Wilcoxon test was used to analyze significant differences in the pneumonectomy and remain lung W/DR levels. A p value of <0.05 was considered statistically significant.
Results
There was no significant difference in preoperative and postoperative hemogram values for subjects in all groups. With the aim of determining whether pulmonary edema developed after pneumonectomy and fluid replacement in subjects in the first group, the weighed lung W/DR results are given in table 1.The initial lung specimen removed with pneumonectomy in the first group had mean wet weight of 3.1366 g (SD ± 1.57). The mean dry weight of pneumonectomy lungs was 0.6175 g (SD ± 0.30). The mean wet weight of the remaining lung was found to be 4.8305 g (SD ± 1.68). The mean dry weight of the remaining lung was identified as 0.9933 g (SD ± 0.38) (Table 1).
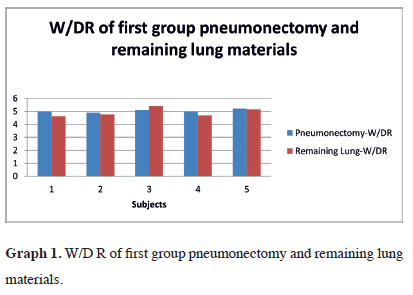
The pneumonectomy and remaining lung W/DR were 5.04 ± 0.11 (19.82%, SD ± 0.41) for pneumonectomy specimens and 4.93 ± 0.34 (20.36% ± 1.35) for remaining specimens (Table 1). The W/DR graph for the first group is shown in graph 1. There was no statistically significant difference between the W/DR in this group (Z = 0.707; p = 0.480).
 Click Here to Zoom |
Graph 1: W/D R of first group pneumonectomy and remaining lung materials. |
In the second group pneumonectomy and remaining lung material was weighed when wet and dry. The mean wet weight of pneumonectomy material was 4.1333 g (SD ± 1.35). The mean dry weight of pneumonectomy material was 0.8147 g (SD ± 0.24). The mean wet weight of remaining lung material was 3.8642 g (SD ± 1.3032) with mean dry weight of 0.7780 g (SD ± 0.24) (Table 2).
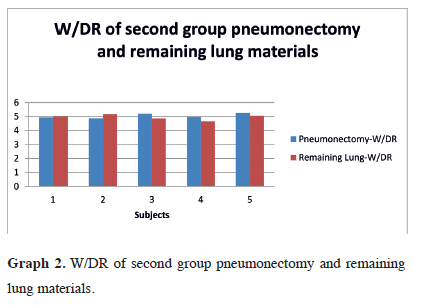
In the second group, pneumonectomy material W/DR was found to be 5.04 ± 0.18 (19.84%, SD ± 0.69) with remaining lung material W/DR of 4.95 ± 0.20 (20.24% SD ± 0/82) (Table 2). The W/DR graph for the second group is observed in graph 2. There was no statistically significant difference found between the materials for W/DR (Z = 0.577; p = 0.564).
 Click Here to Zoom |
Graph 2: W/DR of second group pneumonectomy and remaining lung materials. |
In the third group, the mean wet weight of pneumonectomy material was 4.0437 g (SD ± 1.13), with mean dry weight identified as 0.8071 g (SD ± 0.21). The mean wet weight of the remaining lung was 4.4818 g (SD±1.19) with mean dry weight found to be 0.890 g (SD ± 0.19) (Table 3).
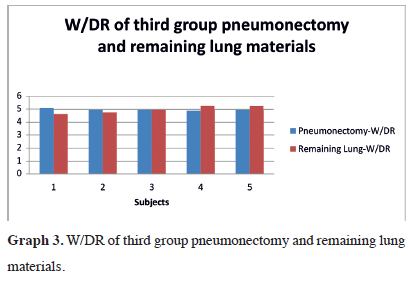
The W/DR of pneumonectomy material in the third group was 5.00 ± 0.08 (19.99%, SD ± 0.31), while it was 4.99 ± 0.28 (19.99%, SD ± 1.13) for remaining lung material (Tables 3). The W/DR variation in the third group is shown in Graph 3. There was no statistically significant difference in the W/DR of the two materials (Z = 0.577; p = 0.564).
 Click Here to Zoom |
Graph 3: W/DR of third group pneumonectomy and remaining lung materials. |
In the fourth group, mean wet weight of pneumonectomy material was 3.127 g (SD ± 0.47) with mean dry weight of 0.630 g (SD ± 0.09). The mean wet weight of the remaining lung material was 3.1038 g (SD ± 1.09) with mean dry weight identified as 0.597 g (SD ± 0.19) (Table 4).
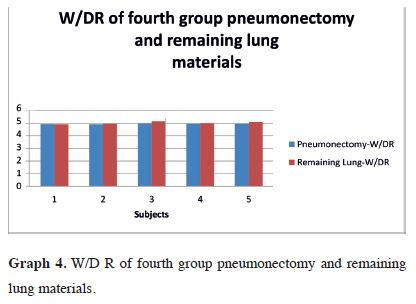
In the fourth group, pneumonectomy material W/DR was 4.95 ± 0.05 (20.17%, SD ± 0.20), with remaining lung material W/DR of 5.02 ± 0.09 (19.90%, SD ± 0.38) (Table 4). The variations in W/DR in the fourth group can be seen in graph 4. There was no statistically significant difference in W/DR in both materials (Z = 0.677; p = 0.498).
 Click Here to Zoom |
Graph 4: W/D R of fourth group pneumonectomy and remaining lung materials. |
The mean W/DR for pneumonectomy material in all isotonic and colloid groups were 5.04 (19.82%, SD ± 0.45), 5.04 (19.84%, SD ± 0.69), 5.00 (20.00%, SD ± 0.31), and 4.95 (20.17%, SD ± 0.20), respectively. There was no statistically significant difference between these ratios (x2 = 2.787; p = 0.426). Again, in the isotonic and colloid groups, the mean W/DR for remaining lung material after pneumonectomy were 4.93 (20.36%, SD ± 1.35), 4.95 (20.24%, SD ± 0.82), 4.99 (20.09%, SD ± 1.13), and 5.02 (19.90%, SD ± 0.38), respectively. There was no statistically significant difference found between these rates (x2 = 2.858; p = 0.414). All results can be seen in table 5. The relationship between the groups can be seen in graph 5.
 Click Here to Zoom |
Graph 5: W/DR in all groups. |
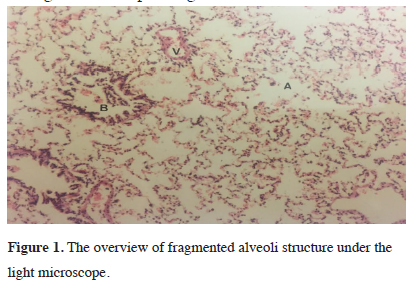
Histopathologic assessment with a light microscope observed common widening of alveoli in all groups. Slight fragmentation of alveoli septa, widening of capillary walls and occasional fragmentation along with minimal hemorrhage within alveoli were observed. As a result, intraalveolar erythrocyte and focal congestion were identified. Additionally, in the interstitial field and normal alveoli, congestion and edema with fluid findings were not encountered. Fragmented alveoli structure due to positive ventilation can be seen under the light microscope in figure 1.
 Click Here to Zoom |
Figure 1: The overview of fragmented alveoli structure under the light microscope. |
Discussion
General anesthetic agents have hypotensive effects. Since Ringer solution (100 L ringer at equal levels to 1 L blood) was identified to be able to carry O2, clear fluid loading was begun in operations [6]. However, later studies have revealed that fluid loading may cause some complications. As a result, investigating the contribution to the development of PE of administration of crystalloid and colloid fluids at normal or high volumes in pneumonectomy carries great importance.For development of PE, meaning filling of alveoli with fluid in experimental and clinical studies, the lung needs to contain 50-100% more fluid than normal [10]). For PE formation, the wet lung/body weight ratio must exceed 0.01 or the upper limit of 0.012 [11]. Additionally, a more certain parameter is the wet/dry ratio (W/DR) of the lung which is normally 5.13 in experimental animals but is accepted as above 5.2 in humans [12]. In this study, the pneumonectomy and remaining lung materials were assessed with W/DR based on values in the literature.
Pneumonectomy and remaining lungs of subjects from the first group had fluid loading at normal volumes and there was no statistically clear increase in W/DR leading to consideration of pulmonary edema. In the second group, in spite of high-volume crystalloid leading after pneumonectomy, there was no statistical difference W/DR in the remaining lung and fluid did not accumulate. In the third group, normal volume of colloids were administered after pneumonectomy, the W/DR in the remaining lung did not increase significantly and congestion was no observed. In the fourth group, high volume of colloid replacement after pneumonectomy did not cause edema in the lung. These results for all groups comply with the literature [9,13].
Zeldin et al. in studies describing non-cardiogenic postpneumonectomy edema administered high volumes of fluids to dogs after pneumonectomy. After this, they identified increased cardiac output and pulmonary artery pressure. As a result, dogs administered excessive fluids developed PE [5]. Especially after right pneumonectomy, the remaining left lung has only 45% of total pulmonary flow and less lymphatic capacity. As a result of high fluid volume loading, there are publications stating edema is caused by increased cardiac output and increased net fluid filtration in pulmonary capillaries [14-16]. Shapira et al. in a publication presenting two cases with PPE identified excessive fluid loading in both cases. They proposed excessive fluid loading was a risk factor [17].
Waller et al. retrospectively screened 402 cases and identified PE in 11 cases. It developed in 5.1% of right pneumonectomies, 4.0% of left pneumonectomies and 1% of lobectomies. Fluids received by patients developing PE were not identified to be excessive when compared to those not developing PE [18]. Again, a study by Turnage and Lunn retrospectively screened 806 pneumonectomy cases and identified PPE in 21. These cases were identified not to have received more fluid than the other pneumonectomy cases [19]. These results comply with our study results.
Lewis et al. identified no associations of right ventricle ejection fraction with pulmonary artery pressure or pulmonary vascular resistance when linked to the pulmonary artery. The volume of pulmonary vascular capacity was revealed to be sufficient to prevent increased pulmonary artery pressure due to increasing volume [20]. Thus, as increasing pulmonary vascular capacity due to excessive fluids can be tolerated, it does not contribute to edema formation.
Lee et al. increased the left atrial pressure in dogs with a balloon catheter. Later they performed pneumonectomy and measured extravasated fluid amounts in the lung. When left atrial pressure was increased by 5 mmHg before and after pneumonectomy, they observed fluid accumulated in the lungs. However, pneumonectomy did not affect fluid accumulation. As a result, they stated that hemodynamic changes did not cause fluid accumulation in the remaining lung after pneumonectomy [21].
Little et al. compared mediastinal lymph node dissection in dogs with pneumonectomy performed to dogs with normal lymph flow. As a result, lymph flow increased up to 10 times; however, this was the limit. If this limit was exceeded, they identified fluid accumulation began in the interstitium of the remaining lung [9]. Additionally, there are studies stating lymph flow is not affected by varying mediastinal lymph node dissection [22]. In our study, the most important reason for edema not forming in the remaining lungs of groups administered high-volume fluids may be adaptation of the lymphatic system.
Assaad et al. stated that though there are publications showing acute lung injury reduced with colloid fluids after lung resection, increasing oncotic pressure may cause renal failure due to kidney hypoperfusion and increase morbidity. They decided the most appropriate regime was colloids along with crystalloids [23].
The lungs are a metabolically active organ. Synthesis of vasoactive agents such as vasoconstrictor agents like angiotensin II or vasodilator agents like bradykinin, kallikrein and prostaglandin occur in the lungs. Van et al. proposed that release of these agents at high volume in the remaining lung after pneumonectomy causes metabolic balance changes [24]. Mathru et al. reported that if edema fluid and serum protein rates >0.6 in patients developing PE after pneumonectomy, it was linked to permeability increase [25]. Leukotrienes, platelet activating factor and some cytokines increase pulmonary capillary pressure without increasing capillary permeability and are proposed to be a major cause of PPE formation [2,7,26]. These results bring to mind the idea that the role of fluids in PPE may be found in metabolic events.
Pang et al. in an experimental study proposed hyperinflation increased fluid flow to the lung [27]. They identified that excessive alveolar distension causes widening of the intercellular interval in capillary endothelium easing fluid and protein leak. Administration of excessive fluids is controversial in PPE; however, they proposed that as there is no third cavity in the chest, fluid limitation is appropriate at present and hyperinflation should be avoided especially in right pneumonectomy to prevent mediastinal shifting [28]. Farkas et al. identified that lying position after pneumonectomy was associated with PPE [29].
In our study, no PE findings were encountered in wet and dry lungs during histopathologic investigation. Sometimes after pneumonectomy, tidal volume apply to both lungs may be applied to a single lung. This may cause barotrauma to the remaining lung. Again, hyperinflation of the remaining lung was identified to cause increased blood flow and pulmonary vascular resistance [26]. Our study assessed this aspect and continuous positive pressure administered to both lungs was administered in the same amount to the single lung after pneumonectomy. The result of this was that though mild levels of alveolar fragmentation, accompanied by minimal hemorrhage in the interstitial area and alveoli was observed histopathologically, as the interstitial field was empty, alveoli did not fill with fluid and edema formation was not observed due to widening. It is considered the pathologies present were linked to hyperinflation. Hyperinflation may cause barotrauma; however, it was revealed not to be sufficient alone to cause PPE formation.
As pulmonary artery pressure, pulmonary vascular capacity, pulmonary capillary wedge pressure and left atrium pressure measurements were not performed, this limits the study of the role of different fluids and doses in PPE formation.
As a conclusion, in all groups administered crystalloid and colloid fluids, at normal and high volume, no significant difference was identified in W/DR of pneumonectomy and remaining lung materials. Histopathologically, all groups had equal amounts of alveolar widening and occasional fragmentation with minimal interstitial and alveolar hemorrhage observed. These histopathological results are caused by hyperinflation. Administration of crystalloid or colloid fluids at normal or high volume is considered insufficient alone to cause PPE etiology in subjects with normal cardiac functions.
Acknowledgments
We greatly appreciate Latife Candan Doğanay, MD. for the histopathologic investigation in this study.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Reference
1) Gibbon JH, Gibbon MH. Experimental pulmonary edema following lobectomy and plasma infusion. Surgery 1942; 12: 694-704.
2) van der Werff YD, van der Houwen HK, van Heesewijk HP, Heijmans PJ, Leusink HA, Duurkens VA, de Boer A. Postpneumonectomy pulmonary edema: a retrospective analysis of incidence and possible risk factors. Chest 1997; 111: 1278-84.
3) Perna AM. Glenn’s Thoracic and Cardiovascular Surgery. Chest 1992; 101: 16-7.
4) Bommart S, Berthet JP, Durand G, Pujol JL, Mathieu C, Marty-Ané C, Kovacsik H. Imaging of postoperative complications following surgery for lung cancer. Diagn Interv Imaging 2017; 98: 11-20.
5) Zeldin RA, Normandin D, Landtwing D, Peters RM. Postpneumonectomy pulmonary edema. J Thorac Cardiovasc Surg 1984; 87: 359-65.
6) Parker JC, Falgout HJ, Parker RE, Granger DN, Taylor AE. The effect of fluid volume loading on exclusion of interstitial albumin and lymph flow in the dog lung. Circ Res 1979; 45: 440-50.
7) Cope DK, Grimbert FR, Downey JM, Taylor AE. Pulmonary capillary pressure: a review. Critic Care Med 1992; 20: 1043-56.
8) Ohkuda K, Nakahara K, Weidner WJ, Binder AN, Staub NC. Lung fluid exchange after uneven pulmonary artery obstruction in sheep. Circ Res 1978; 43: 152-61.
9) Little AG, Langmuir VK, Singer AH, Skinner DB. Hemodynamic pulmonary edema in dog lungs after contralateral pneumonectomy and mediastinal lymphatic interruption. Lung 1984; 162: 139-45.
10) Erdmann AJ, Vaughan TR, Brigham KL, Woolverton WC, Staub NC. Effect of increased vascular pressure on lung fluid balance in unanesthetized sheep. Circ Res 1975; 37: 271-84.
11) Slinger PD. Perioperative fluid management for thoracic surgery: the puzzle of postpneumonectomy pulmonary edema. J Cardiothorac Vasc Anesth 1995; 9: 442-51.
12) Shields TW, editor. General thoracic surgery. Lippincott Williams & Wilkins; 2005.
13) Waller DA, Keavey P, Woodfine L, Dark JH. Pulmonary endothelial permeability changes after major lung resection. Ann Thorac Surg 1996; 61: 1435-40.
14) Hultgren, Herbert N.; Grover, Robert F. Circulatory adaptation to high altitude. Ann Rev Med 1968, 19: 119-152.
15) Chau EH, Slinger P. Perioperative fluid management for pulmonary resection surgery and esophagectomy. Semin Cardiothorac Vasc Anesth 2014; 18: 36-44).
16) Network AR, Wheeler AP, Bernard GR, Thompson BT, Hayden D. Comparison of two fluid-management strategies in acute lung injury. N Engl j Med 2006; 354: 2564-75.
17) Shapira OM, Shahian DM. Postpneumonectomy pulmonary edema. Ann Thorac Surg 1993; 56: 190-5.
18) Waller DA, Gebitekin C, Saunders NR, Walker DR. Noncardiogenic pulmonary edema complicating lung resection. Ann Thorac Surg 1993; 55: 140-3.
19) 19. Turnage WS, Lunn JJ. Postpneumonectomy pulmonary edema: a retrospective analysis of associated variables. Chest 1993; 103: 1646-50.
20) Lewis JW, Bastanfar M, Gabriel F, Mascha E. Right heart function and prediction of respiratory morbidity in patients undergoing pneumonectomy with moderately severe cardiopulmonary dysfunction. J Thorac Cardiovasc Surg 1994; 108: 169-75.
21) Lee E, Little AG, Hsu WH, Skinner DB. Effect of pneumonectomy on extravascular lung water in dogs. J Surg Res 1985; 38: 568-73.
22) Zarins CK, Rice CL, Peters RM, Virgilio RW. Lymph and pulmonary response to isobaric reduction in plasma oncotic pressure in baboons. Cir Res 1978; 43: 925-30.
23) Assaad S, Popescu W, Perrino A. Fluid management in thoracic surgery. Curr Opin Anaesthesiol 2013; 26: 31-9.
24) Van WM, Demedts M. Cardiopulmonary function after lobectomy or pneumonectomy for pulmonary neoplasm. Resp Med 1989; 83: 199-206.
25) Mathru M, Blakeman B, Dries DJ, Kleinman B, Kumar P. Permeability Pulmonary Edema following Lung Resections. Chest 1990; 98: 1216-8.
26) Deslauriers J, Aucoin A, Grégoire J. Postpneumonectomy pulmonary edema. Chest Surg Clin N Am 1998; 8: 611-31.
27) Pang LM, Rodriguez-Martinez FR, Stalcup SA, Mellins RB. Effect of hyperinflation and atelectasis on fluid accumulation in the puppy lung. J Appl Physiol Respir Environ Exerc Physiol 1978; 45: 284-8.



