

2Department of Thoracic Surgery, Gulhane Research and Training Hospital, Ankara, Turkey, Ankara, Turkey
3Department of Thoracic Surgery, Atatürk Chest Disease and Thoracic Surgery Research and Training Hospital, Ankara, Turkey DOI : 10.26663/cts.2020.00014
Summary
Background: Lung cancer is placed in first rows among all types of cancer in both women and men. Treatment options include surgical resection, chemotherapy, and radiotherapy. The main factor, which determines the treatment, is the stage of the disease. Several factors should be considered when assessing the survey ratios of the patients.Materials and Methods: We retrospectively evaluated the patients that we performed pneumonectomy owing to lung cancer from January 2009 to December 2014. Classical and extended pneumonectomies due to non-small cell lung carcinoma were included in the study while excluding the patients who have given neoadjuvant chemotherapy and radiotherapy. We analyzed patients for age, gender, complaints, symptoms, diagnostic steps, pathological specifics, postoperative complications, and surveys.
Results: We evaluated 192 patients retrospectively. The mean age was 58 years. Eight of these patients were female and 184 of them were male. The most common complaint was shortness of breath. We operated 55 patients on right and 137 patients on the left hemithorax. 45 patients were given both chemotherapy and radiotherapy, 79 patients only chemotherapy, 2 patients only radiotherapy, and 66 patients did not take any additional treatment. During the follow-up period, we determined metastases in 15 patients, recurrence in 20 patients, and a second primer tumor in 1 patient. The most common postoperative complication was empyema with fistula. The mean hospital stay was 13 days. 5 years of survival was 35.9%.
Conclusions: The five-year overall survival found to be significantly higher compared to the literature. Despite its unwilling morbidity rate and high mortality rate, pneumonectomy is a surgery to prolong survival in selected cases of lung cancer.
Introduction
Lung cancer constitutes 12-16% of all cancers and is responsible for 18-28% of cancer-related deaths [1]. There are options such as surgery, chemotherapy, and radiotherapy in lung cancer treatment. These treatment methods are applied alone or in combination. The 5-year survival rate of lung cancer was 14% despite improved diagnostic methods, advanced surgical techniques, and non-surgical methods [2].Even though surgery is the most important treatment for non-small cell lung cancer (NSCLC), curative complete resection rate is 20% when diagnosed. For long-term survival, treatment modality can only be decided by a correct staging [3].
In this study, we investigated the factors affecting the survival rate of patients who underwent pneumonectomy for NSCLC.
Methods
Between January 2009 and December 2014, 270 patients underwent pneumonectomy in Ataturk Chest Diseases and Chest Surgery Training and Research Hospital Chest Surgery Clinic. Benign causes, mesotheliomas, and pneumonectomies after neoadjuvant chemotherapy were excluded from the study. While sleeve and completion pneumonectomies are excluded, pneumonectomies that caused by synchronous tumors and extended pneumonectomies were included in the study. In the end, 192 patients were examined retrospectively. Both the data systems of the digital environment and archive files have been examined.Patients’ age at operation time, gender, complaint, background, family history, drug use, PET-CT (positron emission tomography-computed tomography), respiratory function tests, bronchoscopy, preoperative diagnosis, preoperative diagnostic methods, postoperative pathology and staging, chemotherapy, radiotherapy, survival, postoperative hospital stay, development of complications, relapse and metastasis were investigated. Survival information was obtained from the Ministry of Health Death Notification System. The study was approved by the local education committee (No: 2016/529) and was designed in accordance with the principles of the Declaration of Helsinki. The study is the thesis of the first author, and the data of the patients managed between 2009 and 2014 we used.
Statistical Analysis
SPSS 23.0 (IBM Corp., Amonk, NY, USA) was used for statistical analyses. As descriptive statistics, minimum-maximum values and arithmetic mean ± standard deviation and numbers and percentages related to qualitative data were calculated. The conformity of the measurable data to normal distribution was checked with a single sample Kolmogorov-Smirnov test. Mann-Whitney U was used for comparisons between groups and Wilcoxon paired-sample tests were used for comparisons within the group. In qualitative data, Pearson chi-square and single example chi-square tests and Fisher’s exact chi-square tests were used. Survival events were evaluated by Kaplan Meier analyses. P values <0.05 were considered significant.
Results
The mean age of the patients (n = 192) was 58. Patients were between 38 and 80 years old .184 patients were male (95.8%) and 8 were female (4.2%). The most frequent complaint was the shortness of breath with a rate of 35.9%. 10.4% (n = 20) of the patients had no history of using cigarettes, 2.4% (n = 5) of them had used less than 20 packets per year while 72.4% (n = 139) of the patients (n = 28) had used more than 20 packets per year and 14.6% (n = 28) of them had stopped using. While there was no sign of any malignancy in family history in 89.6% of the patients (n = 172), respiratory malignancies were detected in 7.3% (n = 14), family, and non-respiratory malignancies in 3.1% (n = 6). The Charlson comorbidity score was zero in 64.1% of the patients (n = 123).72.9% of the patients (n = 140) were diagnosed by flexible bronchoscopy. The most common lesion was found in the left upper lobe bronchus with 34.4% (n = 66). Left pneumonectomy was performed in 71.4% (n = 137) and right pneumonectomy in 28.6% (n = 55) of all patients. The average hospital stay was 13 days. In 94.8% of patients (n = 182) bronchial surgery border was negative.
While 34.4% (n = 66) of the patients did not receive chemotherapy and radiotherapy, 41.1% received chemotherapy, 1% received radiotherapy and 23.4% received chemoradiotherapy.
One month, 1, 2, 3, 4, and 5 years survival of patients were evaluated. Since not all of the patients completed a 5-year postoperative period, the number of patient groups assessed at 3, 4, 5-year survival rates varied (Table 1).
When we compared the survival times with the operation side, we could not obtain a meaningful result.
Survival was compared according to their histopathological diagnosis. There was no significant difference between the groups (Table 2).
Table 2: Distribution of postoperative histopathologic diagnosis and survival.
Similarly, no statistically significant difference was found between the histopathologic groups by the rates of metastasis, relapse and secondary primer development (p = 0.9) (Table 3).
Table 3: Survival according to recurrence-metastasis-secondary primary status.
A significant difference was observed in the first 30 days when patients with and without chemoradiotherapy were compared (Table 4).
Table 4: Survival by chemotherapy and radiotherapy.
No statistically significant difference was found between the patients who did not develop recurrence or metastasis and those who developed one of these.
When the survival rates of 40 patients who underwent extended resection were examined, the survival rates of 30 days, one, two, three, four, and five years were found 100%, 62.5%, 50%, 36.1%, 32.4%, 32%, respectively. Two of the patients (1%) underwent pneumonectomy due to synchronous tumor. One of the two patients was lost within the first 3 years while the other patient was lost in the fourth year.
The tumor size of 191 of 192 patients could measured in the pathology laboratory and 77.0% of them (n = 147) were over 30 mm and 23.0% of them (n = 44) were 30 mm and below. There was no significant difference between these two groups in terms of the survival rates according to 30-day, 1-year, 2-year, 3-year, 4-year and 5-year. (p = 0.3, p = 0.8, p = 0.3, p = 0.5, p = 0.6, p = 0.5, respectively).
No statistically significant difference was found between sexes in terms of sex-specific survival (Figure 1).
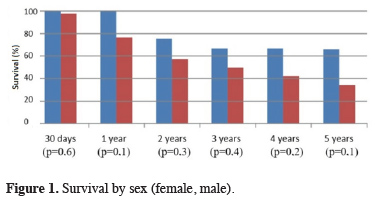 Click Here to Zoom |
Figure 1: Survival by sex (female, male). |
According to the history of smoking, there was no significant difference between the groups in the 30-day, 1-year, 2-year, 3-year, 4-year and 5-year survival of the four groups (p values are p = 0.8, p = 0.5, p = 0.1, p = 0.2, p = 0.7, p = 0.1, respectively).
Relation between the status of bronchial surgical margin and recurrence, metastasis, and secondary primer is shown in Table 5.
Table 5: The relation of the bronchial surgical margin with recurrence/metastasis.
While complications did not develop in 84.6% (n = 154) of patients with bronchial surgical margin negative (n = 182), bleeding in 2.2% (n = 4), empyema in 1.1% (n = 2), fistula in 4.4% (n = 8), fistula in 3.8% (n = 2) wound infections, 0.5% (n = 1) bleeding and fistula, 0.5% (n = 1) tracheoesophageal fistula, 1%, empyema and wound infections in 1 (n = 2), empyema and bleeding in 0.5% (n = 1) developed (p = 0.6).
In comorbidity rating, zero points in 64.1% (n = 123), 1 point in 24.0% (n = 46), 2 points in 9.4% (n = 18) and 3 points in 2.6% (n = 5) were scored. When the comorbidity scores were taken into consideration, there was no significant difference between groups.
In terms of the distribution of patients according to their stages, 8.3% (n = 16) of patients (n = 16) were stage 1a, 13.0% (n = 25) were stage 1b, 23.4% (n = 45) were stage 2a and 18%, 8 (n = 36) stage 2b, 34.4% (n = 66) stage 3a and 2.1% (n = 4) stage 3b. There was no statistically significant difference between the groups in the 30-day, 1-year, 3-year and 5-year survivals, whereas the 2-year survival (p = 0.05) and 4-year survival (p = 0.04) were significantly different between the groups (Table 6).
Table 6: Survival evaluation according to the stage.
In this study, there are stage 1 non-small cell lung cancer patients who performed pneumonectomy because of the localization, intraoperative complication, and accompanied benign status. Despite the histopathological diagnosis was carcinoid in three patients, we performed pneumonectomy due to the main bronchial localization that results in destroyed lung.
When the lymph node involvement of the patients (n = 192) was examined 42.7% (n = 82) were detected as N0, 41.7% (n = 80) as N1 and 15.6% as N2 (n = 30). There was a significant difference between the groups at 1 year, 2 years, 3 years, 4 years and 5 years survival when no difference was detected between the groups in the first 30 days survival.
Metastasis was detected in 7.3% (n = 6) of patients with N0 (n = 82), recurrence was found in 8.5% (n = 7) and second primer was detected in 1.2% (n = 1). Metastasis in 7.5% (n =6) of patients with N1 (n = 80) and recurrence in 10% (n = 8). Metastasis in 10% (n = 3) of patients with N2 (n = 30) and recurrence in 16.7% (n = 5) were found (p = 0.7).
75% of all patients (n = 144) did not have any early or late complications. Bleeding in 4 patients (2.1%), empyema in 2 patients (1.0%), fistula in 8 patients (4.2%), fistula and empyema in 9 patients, 4.7% (0.5%) bleeding and fistula in 1 patient (0.5%), tracheoesophageal fistula in 1 patient (0.5%), empyema and wound infection in 2 patients (1.0%), empyema bleeding was seen. The 30-day, 1-year, 2-year, 3-year, 4-year, and 5-year survival rates of patients with complications were significantly different only in the first 30-day survival (p = 0.0001).
Discussion
Lung cancer is one of the most common causes of death in males and females. Non-small cell lung cancer constitutes more than 80% of all lung cancers [4]. Treatment of early stage non-small cell lung cancer is surgery, while advanced stage and when surgery is not possible non-surgical treatment is in the form of chemotherapy and radiotherapy [5]. The most important factor determining the prognosis is the tumor’s stage [6]. The early diagnosis affects the survival of the patient positively [7].Pneumonectomy for surgical treatment in lung cancer is a form of treatment that is characterized by itself and possible complications increase morbidity and mortality significantly [8].
We retrospectively evaluated 192 patients in our study. The patients were scattered around the age of 60 years. The proportion of male patients is higher than that of female patients.
When the patients were evaluated for 30-day, 1-year, 2-year, 3-year, 4-year and 5-year survival rates, 97.4%, 77.6%, 57.8%, 50%, 43.2% and 35.9% was calculated. In the literature, the early mortality rate was 4.6% and a 5-year survival rate of 27-30% has been reported [9].
When the survival of patients who received chemotherapy, radiotherapy, chemoradiotherapy and did not receive treatment was evaluated, 30-day survival was found to be statistically significant, but additional treatment protocols were applied after 3 weeks postoperatively in a majority of patients, so the effect of chemotherapy, radiotherapy or chemoradiotherapy on survival is controversial. Postoperative chemotherapy and radiotherapy are indicated for survival benefit in the literature [10,11]. When the treatment of patients is being regulated, many factors such as stage and performance are taken into consideration, so that the patient groups show differences within themselves.
In our study, left pneumonectomy was applied to 71.4% (n = 137) of 192 patients and this rate was similar to the literature [12]. There was no significant difference in survival according to the patients’ operation sides. However, the right pneumonectomy mortality is higher in the literature [13].
The 5-year survival rate of the patients without recurrence, metastasis, and second primer was 38.4% while metastasis was 16.7%, recurrence was 20% and the second primer was 100% (p = 0.2). These rates are compatible with the literature [14].
Postoperative pulmonary complications have been reported to increase mortality risk [15]. The 5-year survival rate of patients with complications in our study was found to below.
There was no significant difference between groups according to cell type at 5 years survival (p = 0.09) but the distribution and survival rates of histopathological types are similar when compared with the literature and squamous cell carcinoma is the most common type [16].
When 5-year survival was evaluated according to the postoperative stages of the patients, stage 1a was 50%, stage 1b 10%, stage 2a 43.5%, stage 2b 39.1%, stage 3a 36.8% and stage 3b 0% (p = 0.3). The rate of stage 3 patients was the most common stage, similar to the literature [9]. In a four-year survival analysis, 43.2% (n = 64) of patients survived (n = 148) (p = 0.04). 50% of patients (n = 166) survived over 3 years (n = 83) (p = 0.1). The relationship between stage and 2-year survival was significant (p = 0.05). In the literature, the survival rates of early stage lung carcinomas are quite high and there is an inverse relationship between stage and survival [17].
Survival evaluations can lead to differences in the number of patients at each time slot. This can be explained by the fact that each patient did not complete the five-year postoperative period.
Postoperative complications should be considered when evaluating stage and survival; because the evaluation of survival in early stage and advanced stage patients with complications is different. Comorbidity and performance status should be evaluated together with the stage of the disease. In our study, survival was assessed according to comorbidity of the patients but it was not statistically significant (p = 0.8). The literature has examined the effects of additional diseases and conditions accompanying lung cancer on survival and found no adverse effect on the survival of all [18].
Cigarette smoking, history of respiratory tract benign and malignant diseases, medications, respiratory function status, and the experience and physical conditions of the operating center can also have important effects on survival. When all these factors are evaluated together with the stage, the survival rates may change.
The role of cigarette in lung cancer etiology is known. In our study, the survival benefit of patients was studied by considering the use of 20 cigarettes a year. The 5-year survival rate (45.5%) of the non-smoking patients was found to be higher than that of the current smokers and who quit smoke, as in the literature (p = 0.1) [19].
When the five-year survival rate of 40 patients who underwent extended pneumonectomy was examined, it was observed that mortality was higher than classical pneumonectomy (p = 0.6) [20].
There was no significant result in 5-year survival assessed by considering only the tumor size (p = 0.5). Tumor diameter and survival are inversely proportional in the literature [22].
In our study, no significant difference was found between bronchial surgical margin and survival (p = 0.3). Recurrence was seen in 10% (n = 1) of patients with bronchial surgical margin positive (n = 10) (p = 0.8). In the literature, the recurrence rate increases up to 60% in microscopic bronchial surgery border positivity after lung resections [22]. Complications developed in 2 (20%) of 10 bronchial surgical borderline positive patients (p = 0.6). Postoperative complications have been reported in the literature in bronchial surgical borderline positive resections [23].
When we evaluate the histopathologic type of tumor together with stage, it should be kept in mind that each cell type may give different survival rates at different stages. In our study, survival rates according to cell type were calculated according to time intervals, but cell type and stage relation were not evaluated. When recurrence and metastasis rates were evaluated according to histopathological types, metastasis and recurrence were found in 23.5% and 17.6% of adenocarcinomas. The 50% metastasis rate in the atypical carcinoid group is not statistically significant because of the limited number of patients. It has been reported in the literature that the recurrence rate in non-squamous carcinomas is higher than squamous cell type [24].
In the literature, it is mentioned that recurrence and metastasis rates are higher in N2 than N1 and are also higher in N1 than N0. In our study, 5 year survival according to lymph node involvement was found to be significantly same as with the literature (p = 0.002) [25].
There are some limitations of this study. This study was a retrospective analysis and has a small sample size. Other limitations of this study that it consider parameters like survival, complication, recurrence and metastasis for just over 5 years. To have a deeper understanding of long-term results, patients should be monitored longer. Besides, other outcomes such as quality of life should be considered.
In conclusion, the improvement in patient care services and medical technologies reduced the morbidity and mortality rate in pneumonectomy patients to an acceptable level. Pneumonectomy still is an effective surgical treatment for selected patients in lung cancer.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Reference
1) Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
2) Shields TW: Lung Cancer: Surgical Treatment of Non small cell lung cancer. General Thoracic Surgery, Baltimore, Phiedelphia, London; William and Wilkins, ed: TW Shields; Sixth Edition, Chapter 106;1002-36.
3) Klapper JA, Denlinger CE. Pathologic staging of the mediastinum: When and how? J Thorac Cardiovasc Surg 2014;148:1177-8.
4) Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29.
5) Mathur PN, Edell E, Sutedja T, Vergnon J-M. Treatment of early stage non-small cell lung cancer. Chest 2003;123:176-80.
6) Birim Ö, Kappetein AP, Van Klaveren R, Bogers A. Prognostic factors in non-small cell lung cancer surgery. Eur J Surg Oncol 2006;32:12-23.
7) Robinson E, Mohilever J, Zidan J, Sapir D. Delay in diagnosis of cancer. Possible effects on the stage of disease and survival. Cancer 1984;54:1454-60.
8) Fuentes PA. Pneumonectomy: historical perspective and prospective insight. Eur J Cardiothorac Surg 2003;23:439-45.
9) Ludwig C, Stoelben E, Olschewski M, Hasse J. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non–small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73.
10) Wang EH, Corso CD, Rutter CE, Park HS, Chen AB, Kim AW, et al. Postoperative radiation therapy is associated with improved overall survival in incompletely resected Stage II and III Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2727-34.
11) Liu B, Ding F, Yang S. Progress of postoperative adjuvant chemotherapy in Stage I Non-small Cell Lung Cancer. Zhongguo Fei Ai Za Zhi 2015;18:374-80.
12) Okur E, Kır A, Taşçı E, Keleş M, Yalçınkaya İ, Kutlu CA, et al. Hastanemizde 2006 yılındaki göğüs cerrahisi uygulamaları: 1532 hastanın analizi. Turk Gogus Kalp Dama 2008;16:179-82.
13) Ramnath N, Demmy TL, Antun A, Natarajan N, Nwogu CE, Loewen GM, et al. Pneumonectomy for bronchogenic carcinoma: analysis of factors predicting survival. Ann Thorac Surg 2007;83:1831-6.
14) Kim DJ, Lee JG, Lee CY, Park I-k, Chung KY. Long-term survival following pneumonectomy for non-small cell lung cancer: clinical implications for follow-up care. Chest 2007;132:178-84.
15) Algar FJ, Alvarez A, Salvatierra A, Baamonde C, Aranda JL, López-Pujol FJ. Predicting pulmonary complications after pneumonectomy for lung cancer. Eur J Cardiothorac Surg 2003;23:201-8.
16) Marcus PM, Bergstralh EJ, Fagerstrom RM, Williams DE, Fontana R, Taylor WF, et al. Lung cancer mortality in the Mayo Lung Project: impact of extended follow-up. J Natl Cancer Inst 2000;92:1308-16.
17) Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71.
18) Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Impact of comorbidity on lung cancer survival. Int J Cancer 2003;103:792-802.
19) Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Smoking and lung cancer survival: the role of comorbidity and treatment. Chest 2004;125:27-37.
20) Borri A, Leo F, Veronesi G, Solli P, Galetta D, Gasparri R, et al. Extended pneumonectomy for non–small cell lung cancer: Morbidity, mortality, and long-term results. J Thorac Cardiovac Surg 2007;134:1266-72.
21) Port JL, Kent MS, Korst RJ, Libby D, Pasmantier M, Altorki NK. Tumor size predicts survival within stage IA non-small cell lung cancer. Chest 2003;124:1828-3.
22) Massard G, Doddoli C, Gasser B, Ducrocq X, Kessler R, Schumacher C, et al. Prognostic implications of a positive bronchial resection margin. Eur J Cardiothorac Surg 2000;17:557-65.
23) Wind J, Smit EJ, Senan S, Eerenberg J-P. Residual disease at the bronchial stump after curative resection for lung cancer. Eur J Cardiothorac Surg 2007;32:29-34.



