

2Department of Pathology, Bursa City Hospital, Bursa, Turkey
3Department of Thoracic Surgery, University of Health Sciences, Yedikule Chest Diseases and Thoracic Surgery Application and Research Center İstanbul, Turkey DOI : 10.26663/cts.2021.0005
Summary
Background: Complete surgical resection has been the gold standard in the treatment of lung cancer. Intrapericardial approach may be required to resect the advanced stage tumors completely. This study aims to compare the morbidity and mortality rates of intrapericardial and standard pneumonectomies.Materials and Methods: The records of patients operated on due to non-small cell cancer between the years 2001 and 2008 were evaluated retrospectively. The data including age, sex, respiratory function tests, tumor site, cell type, stage, resection type, complications, mortality and survival rates were recorded. The morbidity, mortality and survival rates of intrapericardial and standard penumonectomies were compared.
Results: The study included 126 cases with pneumonectomy (90 standard, 36 intrapericardial) with mean age of 56.6 ± 8.42 years. Major morbidity rates of intrapericardial and standard groups were 30.6% and 21.1% respectively, and the difference was not significant (p = 0.233). Mortality rates of intrapericardial and standard groups were 8.3% and 8.9% respectively, and the difference was not significant (p = 0.533). The mean survival of all patients was 24.13 ± 3.13 months. The survival rate was significantly in the intrapericardial group compared to standard group (23 months vs 32 months, p = 0.03).
Conclusions: Intrapericardial approach is a safe method in the treatment of locally advanced lung cancer without increasing morbidity or mortality.
Introduction
Despite developing technology and struggle lung cancer has kept still its rank as one of the deadliest cancers [1]. The stage of the disease and the physical characteristics of the patient determine the strategy of the treatment. A complete surgical resection is superior to both chemotherapy and radiotherapy. Inoperability has decreased to acceptable rates by the improvements in staging and imaging modalities.The surgical dissection especially is difficult in centrally located tumors, tumors invading pulmonary artery or veins, in the presence of lymph nodes attacking the hilar structures, hilar fibrosis or previous dissections. Opening the pericardium may usually be necessary in these situations to control the hilar vessels [2,3]. It also helps to access the proximal pulmonary artery and superior vena cava (SVC) when the centrally located tumor invade the pericardium.
The aim of the current study is to analyze the morbidity and mortality in patients who underwent pneumonectomy between 2001 and 2008 due to non-small cell lung cancer (NSCLC) by comparing the results of patients with standard pneumonectomy to pneumonectomy performed through the intrapericardial approach.
Methods
The medical records of all patients with resections due to non-small cell lung cancer between 2001 and 2008 were evaluated retrospectively. The age, sex, pulmonary function tests (vital capacity and forced expiratory volume in the first second), location, histopathology and stage of the tumor, type of resection, morbidity, mortality, and survival of the cases were noted. Patients with incomplete resections, completion pneumonectomies, N2 disease detected by mediastinoscopy and cases with incomplete data were excluded from the study.A thorough physical examination, routine biochemical tests, pulmonary function tests, arterial blood gas analysis, and fiberoptic bronchoscopic examination were performed in all cases. Computed thoracic tomography (CT) was performed in all cases to better localize the tumor and evaluate the mediastinal lymph nodes. The histopathological diagnosis was reached by either transbronchial or trans-thoracic needle biopsy. A positron emission tomography (PET/CT) was performed to exclude a distant metastasis and detect mediastinal lymph node invasion since 2003. In centrally located tumors and patients with tumors over 3 cm a cranial magnetic resonance imaging (MRI) was performed.
Before surgery, all patients underwent pulmonary function tests to calculate the FEV1 and a perfusion scintigraphy was performed in patients with FEV1 less than 800 mL. A pO2 over 60 mmHg and pCO2 less than 45 mmHg were the limits of arterial blood gas analysis for performing the surgical resection. Patients with a history of heart disease, active chest pain, dysrhythmia, or age over 70 were referred to a cardiologist assess the cardiac risk. The cardiac risk was assesed by the consultant cardiologists according to the ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery. To detect the situation of the mediastinal lymph node standard or extended mediastinoscopy was performed in all cases. Patients with N2 disease were referred to oncology for neoadjuvant therapy, and with N3 disease for definitive chemotherapy. The pathology reports were examined, and patients were re-staged according to 8th TNM staging system reported by the international association of the study of lung cancer (IASLC) [4].
Standard posterolateral thoracotomy was performed in all cases after rigid bronchoscopy and double lumen endotracheal intubation under general anesthesia. Pulmonary vessels were stapled in the standard group while they were sutured with non-absorbable sutures in the intrapericardial group. Bronchi were closed with stapler in all cases. A prolene mesh was used when necessary to restore the pericardial defects. The opening of the pericardium was not routine. However, in cases where either pulmonary veins or artery was invaded intrapericardially, or presence of invasion of the superior vena cava or atrium then the pericardium was opened. Furthermore, when the hilar dissection was not possible due to fibrotic lymph nodes or dense adhesion the pericardium was opened. Chest wall resection was added to the planned procedure in cases with tumors invading the chest wall.
After the operation, all cases were monitored in the intensive care unit for at least 24 hours and patient-controlled analgesia was provided. Patients were seen at the outpatient unit after discharge every 3 months for the first 2 years and every 6 months then on to detect any possible recurrences. All minor and major complications were noted. Arrhythmias of any kind that deteriorated the general condition of the patient and required medical treatment were accepted as major complication.
Exitus in the first 30 days and 90 days was accepted early and late mortality rates, respectively. Survival data were provided from either outpatient records or phone calls to the patients or their relatives. The study was approved by the institutional review board (No: 2020-31) and was conducted in accordance with the principles of the Declaration of Helsinki.
Statistical Analysis
Categorical variables were depicted as percentages and frequencies while continuous variables as means and standard deviation. Chi square test was used to compare the categorical data and Student’s t-test was for comparing the means of the groups. To calculate the survival Kaplan-Meier survival analysis and for comparing the groups log-rank test were performed. Because the statistical calculations were based on the data of all cases included in the study period, the power analysis was not performed. IBM SPSS (version 15.0, SPSS Inc. Chicago, Illinois, United States) was used to perform all calculations and p < 0.05 was accepted as statistical analysis.
Results
During the study period a total of 393 patients underwent pulmonary resection, and 126 out of these were pneumonectomy. The remaining cases were lobectomy (201), segmentectomy (18), completion pneumonectomy (6), incomplete resection (12), N2 or N3 patients (57), and lost follow-up (23) and not included in the statistical analyses. Out of 126 pneumonectomy cases 36 were performed through the intrapericardial approach. This group was compared to remaining 90 cases with standard pneumonectomy with respect to morbidity, mortality, and survival.Forty-two of the cases were male and the mean age of all cases was 56.6 ± 8.42 (Table 1). The rate of patients with neoadjuvant therapy was higher in the intrapericardial group but the difference did not reach statistical significance. Moreover, the tumor stage was more advanced in the intrapericardial group than the standard group.
Table 1: Clinical characteristics of the patients.
Arrythmias were the most frequent symptom in both groups whereas bronchopleural fistula was the most mortal complication (Table 2). Major morbidity rate was calculated in the intrapericardial group and the standard group 30.6% (11 cases) and 21.1% (19 cases), respectively, but the difference did not reach statistical significance (p = 0.263). The treatment of arrythmias was provided by the consultant cardiologist of the hospital. A total of seven cases developed postoeprative hemorhage and all required thoracotomy to control the source of bleeding. The most demanding complication was bronchopleural fistula experienced in 17 cases. The initial intervention was tube thoracostomy and appropriate antibiotics in all cases. The fistula was minor and responded to the teratment in seven cases. Two out of 10 remaining cases underwent omentoplasty in the early postoperative period. Thoracostoma was performed in the remaining eight cases. After serial debridment of the cavity omentoplasty was added in the cases. Two cases were not eligible for omentoplasty and followed at outpatient debriment and wound dfessing. In cases with pneumonia apropriate antibiotics were given and pulmonary physiotherapy was also added. Mechanical ventilation was added to the treatment when the patient developed respiratory failure.
Three cases (8.3%) in the intrapericardial group died during the first month of operation. One of the two cases who had right pneumonectomy and partial vena cava superior resection died from thrombus development in the SVC, and the other died from cerebral emboli. The last patient with left pneumonectomy in this group died from pulmonary embolism. In the standard group the rate mortality in the first 30 days was 8.9% (8 cases).
Table 2: The disribution of the complications.
Six cases died from pneumonia and respiratory failure because of bronchopleural fistula development and the remaining 2 died from myocardial infarction. The difference between the mortality rates of the groups was not statistically significant (p = 0.533). Univariate analysis showed that right pneumonectomy affected both morbidity and mortality when compared to left pneumonectomy (p = 0.002).
The mean survival of all cases was 24.13 ± 3.13 months (Figure 1). Intrapericardial and standard groups had 23 ± 6.37 months and 32 ± 5.53 months of mean survival times, respectively, and the difference was found statistically significant (p = 0.030). In survival analysis age (over 60, 21 months vs younger 60, 37 months) and advanced tumor stage (stage 3, 22 months vs stage 2, 39 months) were found to significantly affect the survival adversely (p = 0.001).
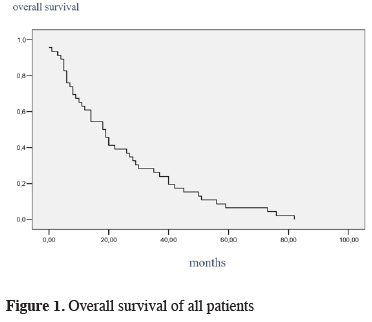 Click Here to Zoom |
Figure 1: Overall survival of all patients |
Discussion
Complete resection is sine qua non in the treatment of lung cancer surgery. To reach this goal extended resections are required when necessary [5,6]. These extended resections carry more morbidity and mortality when compared to standard procedures [7,8]. Hilar dissection is especially difficult in the presence of centrally located tumors invading pulmonary artery or veins, attacked and fixed lymph nodes preventing the vascular dissection, and previous hilar dissection. These situations require intrapericardial approach to control the vascular structures to make complete resection possible [9]. Allison reported that intrapericardial approach was especially safer and faster in the treatment of tumors invading the pericardium in 1946 [10]. Later, in 1948 Brock reported intrapericardial approach (Block dissection pneumonectomy) as more curative operation [11].In this series the most frequent indication for opening the pericardium was the invasion of the pulmonary veins like many series in the literature [9,12,13]. In contrast, Dartavelle et al. reported on 30 cases of T4 tumors invading the superior vena cava [14]. In the recent decades some advanced staging techniques, transesophageal echocardiography (TEE), and videopericardioscopy (VPC), have been used to better delineate the intrapericardial invasion and to prevent futile thoracotomies and incomplete resections [15-17]. During the study period TEE was unavailable and videoperioscopy was rarely performed. The superior vena cava invasion ranked the 5th place and a graft was used to restore the continuity.
The morbidity and mortality rates of pneumonectomies performed due to lung cancer has been reported as low as 24% to 59% and from 3% to 12% respectively [7,18,19]. Although right pneumonectomies have higher morbidity and mortality rates it was rarely reported that intrapericardial pneumonectomies carried higher rates compered to standard approach. Intrapericardial pneumonectomies had acceptable morbidity and mortality rates despite of higher supraventricular arrythmia rates. Krowka et al reported that the reason for postoperative arrythmias were pericardial irritation, intrapericardial ligation of the pulmonary vessels, extended resections, longer operation time, and atrial injury [20]. In a large series with 259 cases Foroulis et al reported that right pneumonectomy over left and intrapin this serieericardial pneumonectomy over standard pneumonectomy had higher arrhythmia risk [21]. The overall morbidity rate in this series was around 24% and was at the lower edge of the literature. The possible reason could be the definition and classification of the complications were not uniform. Whereas the overall mortality of the study was 8.7% and was close to the upper limit of the study. The advanced tumor stage and high rate of right pneumonectomy could explain the relatively high rate of mortality.
The most challenging complication after pneumonectomy is the development of bronchopleural fistula. The risk of BPF has been reported between 2.1% and 8%, and intrapericardial ligation of the vessels did not increase the risk [22]. In this study the rate of BPF was higher than the literature, and, the risk was found higher in the intrapericardial group besides. Existence of more advanced tumor stage and higher number of right pneumonectomy in this group might explain this discrepancy. Furthermore the study was performed in a teaching hospital. The operations were performed by various surgeons and fellows with different experiences.
Pricopi et al reported on that the right side, advanced age, and advanced tumor stage increased the mortality whereas the extent of the resection did not change it in a series with 2064 cases [19]. Similarly, Park et al pointed out that pneumonectomy had higher mortality rates when compared to lesser resections, but he also reported that intrapericardial approach did not affect the mortality rate significantly [23].
The five-year survival of non-small cell lung cancer was reported between 21% and 31% in the literature [24,25]. In a series consisting of 55 pneumonectomies with stage IIIA overall survival was found 29%, and interestingly 42% of the group had 20% five-year survival rate despite of N2 metastases [26]. Riquet et al reported on 32% five-year survival rate in a large series consisting of 1466 patients of whom 342 had extended pneumonectomy [27]. In that study advanced age, advanced tumor stage, extended pneumonectomy, and adenocarcinoma adversely affected the survival, but right pneumonectomy did not affect the survival when compared to left side. In the current study the five-year survival rate was found 21%. The possible reason for this relatively low survival rate could be higher number stage III cases than stage II. Similarly, advanced age and advanced tumor stage adversely affected the survival. The reason for lower survival rate in the intrapericardial group could be higher tumor stage in the group.
Limitations of the study
The retrospective nature of the study, limited number of patients, insufficient number of cases representing the subgroups, and inhomogeneity of the groups with respect to tumor stage were the limitations of the current study. Moreover, the study comprises limited number of variables affecting the morbidity, mortality, and the survival. Other limitations of the study might be missing clinical characteristics of the cases like TNM subsets and insufficiencies in the follow up such as local and systemic recurrences.
As a conclusion, intrapericardial dissection could be necessary in the treatment of locally advanced non-small cell lung cancer to reach a complete resection with all negative tumor margins. Invasion of proximal pulmonary vessels, pericardium or left atrium may adversely affect the prognosis, but they do not affect the operability. Though intrapericardial approach increase the supraventricular arrythmia rates it can be performed with acceptable morbidity and mortality rates.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Ethics approval
The study was approved by the Ethics Committee of Health Sciences University (No: 2020-31).
Authors’ contributions
AP; Conceived and designed the analysis, collected the data, contributed data/analysis tools, performed the analysis, co-wrote the paper, MN; contributed data/analysis tools, co-wrote the paper MM; co-wrote the paper.
Reference
1) Kara F, Ilter H, Keskinkilic F. Turkish Cancer Statistics 2015, Ministry of Health. Ankara 2018; 1-64.
2) Hasdiraz L, Oguzkaya F, Bilgin M. An evaluation of pulmonary resections accompanied by intrapericardial ligation for pulmonary cancers. Turkish J Thorac Cardiovasc Surg 2007; 15: 123-6.
3) Olcmen A, Kutlu A, Dincer I, Bekar Y, Olcmen M. Intrapericardial approach in lung resections (27 cases). Turkish J Thorac Cardiovasc Surg 1996; 4: 109-13.
4) Sobin LH, Wittekind C. International Union Against Cancer (UICC) TNM classification of malignant tumours. 6. New York: Wiley; 2002.
5) Loscertales J, Jime´nez-Mercha´n R, Congregado- Loscertales M, Arenas-Linares C, Giro´ n-Arjona JC, Arroyo Tristan A et al. Usefulness of videothoracoscopic intrapericardial examination of pulmonary vessels to identify resectable clinical T4 lung cancer. Ann Thorac Surg 2002; 73: 1563-6.
6) Bernard A, Deschamps C, Allen MS, Miller DL, Trastek WF, Jenkins GD et al. Pneumonectomy for malignant disease: factors affecting early morbidity and mortality. J Thorac Cardiovasc Surg 2001; 121: 176-82.
7) Harpole DH Jr, DeCamp MM Jr, Daley J, Hur K, Oprian CA, Henderson WG, Khuri SF. Prognostic models of thirty-day mortality and morbidity after major pulmonary resection. J Thorac Cardiovasc Surg 1999; 117: 969-79.
8) Stephan F, Boucheseiche S, Hollande J, Flahault A, Cheffi A, Bazelly B, Bonnet F. Pulmonary complications following lung resection. A comprehensive analysis of incidence and possible risk factors. Chest 2000; 118: 1263-70.
9) Spaggiari L, D’Aiuto M, Veronesi G, Pelosi G, de Pas T, Catalano G et al. Extended pneumonectomy with partial resection of the left atrium without cardiopulmonary bypass for lung cancer. Ann Thorac Surg 2005; 79: 234-40.
10) Allison PR. Intrapericardial approach to lung root in the treatment of bronchial carcinoma by dissection pneumonectomy. J Thorac Surg 1946; 15: 99.
11) Johnson J, Kirby CK, Blakemore WS. Should we insist on radical pneumonectomy as a routine procedure in the treatment of carcinoma of the lung? J Thorac Surg 1958; 3: 309.
12) Rendina EA, Venuta F, Ibrahim M. Intrapericardial pneumonectomy. Multimed Man Cardiothorac Surg 2006; (109): mmcts.2004.000091.
13) T Fukuse, H Wada, S Hitomi. Extended operation for non-small cell lung cancer invading great vessels and left atrium. Eur J Cardiothorac Surg 1997; 11: 664-9.
14) Dartevelle PG, Mitilian D, Fadel E. Extended surgery for T4 lung cancer: a 30 years’ experience. Gen Thorac Cardiovasc Surg 2017; 65: 321-8.
15) Wang KY, Lin CY, Kuo-Tai J, Yuan L, Chang HJ. Use of transesophageal echocardiography for evaluation of resectability of lung cancer. Acta Anaesthesiol Sin 1994; 32: 255-60.
16) Loscertales J, Jime´nez-Mercha´n R, Congregado- Loscertales M, Arenas-Linares C, Carlos Giro´ n-Arjona J, Arroyo Tristan A et al. Usefulness of video thoracoscopic intrapericardial examination of pulmonary vessels to identify resectable clinical T4 lung cancer. Ann Thorac Surg 2002; 73: 1563-6.
17) Pompeo E, Tacconi F, Mineo TC. Flexible videopericardioscopy in cT4 nonsmall-cell lung cancer with radiologic evidence of proximal vascular invasion. Ann Thorac Surg 2007; 83: 402-8.
18) Ginsberg RJ, Hill LD, Eagan RT, Thomas P, Mountain CF, Deslauriers J et al. Modern thirty-day operative mortality for surgical resections in lung cancer. J Thorac Cardiovasc Surg 1983; 86: 654-8.
19) Pricopi C, Mordant P, Rivera C, Arame A, Foucault C, Dujon A et al. Postoperative morbidity and mortality after pneumonectomy: a 30-year experience of 2064 consecutive patients. Interact Cardiovasc Thorac Surg 2015; 20: 316-21.
20) Krowka MJ, Pairolero PC, Trastek VF, Payne WS, Bernatz PE. Cardiac dysrhythmia following pneumonectomy. Chest 1987; 9: 490.
21) Foroulis CN, Kotoulas C, Lachanas H, Lazopoulos G, Konstantinou M, Lioulias AG. Factors associated with cardiac rhythm disturbances in the early post-pneumonectomy period: a study on 259 pneumonectomies. Eur J Cardiothorac Surg 2003; 23: 384.
22) Deschamps C, Bernard A, Nichols FC 3rd, Allen MS, Miller DL, Trastek VF et al. Empyema and bronchopleural fistula after pneumonectomy: factors affecting incidence. Ann Thorac Surg 2001; 72: 243.
23) Park B, Cho JH, Kim HK, Choi YS, Zo JI, Shim YM, Kim J. Long-term survival in locally advanced non-small cell lung cancer invading the great vessels and heart. Thorac Cancer 2018; 9: 598-605.
24) Ramnath N, Demmy TL, Antun A, Natarajan N, Nwogu CE, Loewen GM et al. Pneumonectomy for bronchogenic carcinoma: analysis of factors predicting survival. Ann Thorac Surg 2007; 83: 1831–6.
25) Deslauriers D, Ugalde P, Miro S, Deslauriers DR, Sylvie Ferland S, Bergeron S et al. Long-term physiological consequences of pneumonectomy. Semin Thoracic Surg 2011; 23:196-202.



