

Summary
Background: Video-assisted thoracoscopic surgery (VATS) has become the standard treatment method for pleural empyema’s surgical treatment. Postoperative pain, length of hospital stay, and cosmetic results are satisfactory, especially with the uniportal approach. In this study, we retrospectively evaluated and compared outcomes of patients treated with uniportal and biportal VATS.Materials and Methods: A total of 73 patients who underwent VATS for empyema in our clinic between January 2017 and October 2020 were retrospectively evaluated. Patients who underwent uniportal and biportal VATS were compared in terms of age, sex, side and stage of empyema, length of hospital stay, comorbidities, smoking history, American Society of Anesthesiologists (ASA) physical status score, number of chest tubes placed postoperatively, postoperative Heimlich valve placement, postoperative complications, postoperative drainage volume, recurrence, preoperative and 72-h postoperative C-reactive protein (CRP) and white blood cell (WBC) values, and postoperative 24-h and 72-h VAS (visual analog scale) pain scores. Univariate comparisons were done with R-based Jamovi statistical software.
Results: Fifty-two (71.2%) of the patients were male, and the mean age was 57 (IQR 41-67). Empyema was stage 2 in 38 (52.1%) patients and stage 3 in 35 (47.9%) patients. VATS was uniportal in 52 patients (71.2%) and biportal in 21 patients (28.8%). The uniportal VATS group had significantly lower drainage volume (p = 0.006) and VAS scores (p < 0.001). There were no statistical differences in the other parameters.
Conclusions: Our data indicate that uniportal VATS was superior to biportal VATS for the treatment of empyema in terms of postoperative pain and drainage.
Introduction
Pleural empyema is defined as the presence of pus in the pleural space or positive Gram staining of pleural effusion and/or isolation of a microorganism in pleural fluid culture [1]. It usually occurs secondary to pneumonia, and morbidity and mortality rates are between 2% and 30% [2]. Other causes are surgery, thoracic interventions, esophageal diseases, and abdominal sepsis [3].Parapneumonic effusion develops in 14% to 19% of patients with community-acquired pneumonia, and approximately one-third of these cases occur as complicated parapneumonic effusion or empyema [4,5]. The American Thoracic Society classified pleural empyema in three stages: stage 1 is the exudative stage, stage 2 is the fibrinopurulent stage, and stage 3 is the organizational stage [6]. The British Thoracic Society recommends drainage (thoracentesis/tube thoracostomy) and medical treatment for stage 1 empyema. In contrast, for stage 2 and stage 3 cases, removal of debris and purulent tissues to enable re-expansion is recommended, with deloculation and decortication if necessary [7]. Thoracotomy and video-assisted thoracoscopic surgery (VATS) are the preferred approaches when surgical treatment of empyema is required, with VATS yielding satisfactory results [7,8]. Different publications on this popular topic have concluded that the VATS technique is effective and safe for treatment regardless of the stage [9-11]. Early surgical intervention was also shown to significantly reduce the length of hospital stay, costs, and mortality and morbidity rates, especially in patients with stage 2 and stage 3 empyema [12]. A few studies in the literature compare the effectiveness and outcomes of U-VATS and B-VATS in pneumothorax patients [13-15]. There are also reports in the literature of the results and features of lung resection performed with U-VATS [16,17].
Our study is the first to compare U-VATS and B-VATS in the treatment of empyema; our paper retrospectively evaluates the efficacy and clinical outcomes of empyema treated with uniportal VATS (U-VATS) and biportal VATS (B-VATS) techniques.
Methods
This retrospective chart review included 73 patients who underwent VATS for empyema between January 2017 and October 2020. In this period 324 VATS performed for different indications. Five patients with missing data were not included (Figure 1). The patients’ age, sex, side and stage of empyema, length of hospital stay, comorbidities, smoking history, American Society of Anesthesiologists (ASA) physical status score, surgical technique (U-VATS or B-VATS), postoperative Heimlich valve placement, number of chest tubes placed postoperatively, postoperative complications, postoperative drainage volume, recurrence, preoperative and 72-h postoperative C-reactive protein (CRP) and white blood cell (WBC) values, and visual analog score (VAS) at postoperative 24 and 72 hours were analyzed. These parameters were compared between the U-VATS and B-VATS groups.
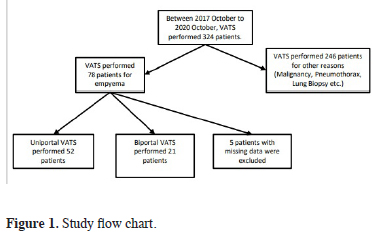 Click Here to Zoom |
Figure 1: Study flow chart. |
ASA scores were obtained from the anesthesiologist’s consultation notes, and VAS scores were obtained from the patients’ follow-up charts.
All patients were evaluated with chest computed tomography (CT) and ultrasound (US). Pleural fluid and/or sputum culture samples were collected from all patients, and empiric broad-spectrum antibiotic therapy was administered until the results were obtained. Specific antibiotic treatment was administered according to culture results.
During this period, if the amount of free fluid detected in chest CT or US was 1000-1500 mL or more, drainage was performed by tube thoracostomy. VATS was performed in patients who could not be re-expanded and showed no clinical improvement after drainage and those with intense septation and fluid volume less than 1000 mL [18]. All operations were initiated as uniportal, either using the existing drain incision or through an incision made at the site of the most significant free fluid detected on chest US. In necessary cases (e.g., difficult manipulation), a second entry site was made, and the procedure was completed as biportal. After deloculation and complete removal of the tissue debris, patients whose lungs were easily expanded were evaluated as stage 2, while those who had limited ventilation and incomplete expansion and required decortication were evaluated as stage 3. Patients with impaired postoperative lung expansion (incomplete expansion despite decortication due to intraparenchymal infection severity) and immobile patients (e.g., home care, neurological patients) were discharged with Heimlich valve and followed up. Approval for this retrospective, descriptive, single-center study was obtained from the Clinical Research Ethics Committee of Recep Tayyip Erdogan University Faculty of Medicine (decision no: 2020/193, dated 03.09.2020).
VATS Technique
All surgical procedures were performed under general anesthesia with one-lung ventilation using a selective double-lumen endotracheal tube. The patients were positioned in lateral decubitus with the upper arm suspended in flexion and abduction. The day before the procedure, chest US was performed at the bedside with patients in the lateral decubitus position, and the site of most abundant loculation or fluid was marked, preferably on the midaxillary line. Chest exploration was performed with a 10-mm 30° thoracoscope introduced through an incision at the marked location or through the existing drain incision if present. Adhesions at the entry site were bluntly dissected using fingers and endoscopic instruments to create space. If this space was considered suitable after exploration, the incision was extended to 3 cm, and a tissue-protective retractor (Alexis O wound protect/retractor) was placed. The endoscopic instruments were introduced through this port, and sharp and blunt dissections were performed. If space was not considered adequate for dissection and decortication and manipulation was difficult, a second port incision was made at an appropriate site determined intraoperatively.
The procedures started with rupturing the septa (deloculation), debridement, and evacuation of the effusion. All infected tissues on the pleural surfaces, diaphragm, and sinuses were gently cleared using ovarian forceps. Effusion and tissue samples were obtained for simple culture, tuberculosis culture, cytology, and pathologic examination. Adhesions were separated by sharp and blunt dissection from the diaphragm to the apex. The thoracic cavity was washed several times with warm saline and cleared of infectious tissue and fluid residues by aspiration. After the lung tissue was released within the thoracic cavity, the parietal pleura’s thickened sections were decorticated using Kelly forceps and gauze balls. The affected areas of the visceral pleura were detected by venting the lung. While the lung was ventilated at a low volume, the visceral pleura was separated from the lung surface at a suitable site using Kelly forceps, gauze balls, and ovarian forceps. Starting dissection from this site, all affected pleural surfaces were decorticated. No intervention was performed for minimal leaks from lacerated parenchymal surfaces. Deeply lacerated parenchymal surfaces with massive leaks were repaired by primary suture or reinforced with tissue adhesive.
Local analgesia with 3 mL of 0.5% isobaric bupivacaine (5 mg/mL) was administered by thoracoscope at two intercostal levels above and below the utility incision and at the drain incision. At the end of the procedure, in most cases, a single chest drain (preferably 32 F) was placed after confirming the lung was fully ventilated and expanded. Patients who were expected to have more prolonged postoperative expansion and recovery time received two drains, a 32 F tube at the basal position and 28 F at the apex.
The decision to remove the chest tube was made by evaluating the patient’s medical treatment duration, radiological evidence of lung expansion, clinical condition (fever, dyspnea, deterioration of inflammatory markers), decrease of daily drainage below 100 mL and absence of air leak. Clinically stable patients had completed medical treatment and showed normal inflammatory parameters but had lung expansion defect, those with persistent air leak (even minimal), and home care (bed-bound) patients were discharged with a Heimlich valve. These patients’ drains were removed upon demonstration of clinical and radiological improvement during follow-up.
The mean postoperative follow-up time of the patients was 141 days. Contrast-enhanced chest CT scans were performed at 3rd and 6th months of follow-up.
Statistical Analysis
R-based Jamovi statistical software (Version 1.6.5 for Mac OS; https://jamovi.org) was used for statistical analysis. The distribution characteristics of continuous data were evaluated using the Shapiro-Wilk test, and Q-Q plots. Non-normally distributed data were expressed as the median and interquartile range (25th percentile-75th percentile) and compared between the U-VATS and B-VATS groups using the Mann-Whitney U test. Categorical variables were reported as number (n) and frequency (%) and compared between the groups using Pearson’s chi-squared (χ2) test. A statistical significance level of p < 0.05 was used for all tests.
Results
Fifty-two (71.2%) of the patients were male, and the mean age was 57 (IQR 41-67). Twenty-seven patients (36.9%) were smokers. In terms of comorbidities, 10 patients (13.7%) had chronic obstructive pulmonary disease, 20 (27.4%) had ischemic heart disease, 12 (16.4%) had hypertension, and 9 (12.3%) had diabetes. Empyema was on the right side in 49 patients (67.1%) and was stage 2 in 38 patients (52.1%) and stage 3 in 35 patients (47.9%). Preoperative ASA score was ASA-2 in 25 patients (34.2%), ASA-3 in 31 patients (42.5%), and ASA-4 in 17 patients (23.3%).The most common symptoms were fever in 46 (63.0%), cough in 25 (34.2%), dyspnea in 9 (12.3%), and chest pain in 7 patients (9.6%). Empyema occurred due to bronchopleural fistula secondary to lung cancer in 1 patient, while all other patients had complicated parapneumonic effusion.
A total of 52 patients (71.2%) underwent U-VATS, and 21 (28.8%) underwent B-VATS. A single chest drain was placed in 54 patients (74.0%), while 19 patients (26.0%) had two chest drains. Nine (12.3%) of the patients were discharged with a Heimlich valve, and 64 (87.7%) were discharged after drain removal. The median postoperative follow-up time was 141 (IQR 105-176) days (Table 1).
Table 1: Demographic and clinical characteristics of the patients (n = 73).
The patients were divided into uniportal and biportal groups and compared in terms of age, sex, smoking, empyema side, drainage, recurrence, preoperative and postoperative CRP (mg/L; normal range 0-5 mg/L), preoperative and postoperative WBC (×103/uL), empyema stage (stage 2 or 3), preoperative and postoperative hospital stay, discharge with Heimlich valve, number of chest tubes placed postoperatively, VAS at postoperative 24 and 72 h, and procedure times. Of these findings, U-VATS was only beneficial in terms of drainage volume and VAS scores (p < 0.001 and p = 0.006, respectively). There were no statistical differences in the other characteristics (Table 2).
The only mortality occurred in the patient with empyema due to bronchopleural fistula secondary to lung cancer. This patient underwent B-VATS and died in the intensive care unit due to poor general condition during postoperative follow-up. Empyema recurred in 3 patients, of whom 2 were treated with re-VATS, and 1 underwent open decortication (OD) in the early period. Two patients with relapse underwent U-VATS, and the other underwent B-VATS. Both patients who underwent re-VATS were in the uniportal group, and a new port incision was not required in the second operation. No late relapse was observed. In terms of minor complications, inadequate expansion persisting longer than 5 days was observed in 2 patients (9.5%), anemia in 2 patients (2.7%), air leak persisting for longer than 5 days in 3 patients (8.1%), and surgical site infection in 1 patient (4.1%) (Table 3).
Discussion
Surgical treatment is currently the standard approach for stage 2 and 3 empyema. VATS decortication (VATSD) and OD are the preferred techniques for this purpose. Recent publications have discussed the superiority of VATS in the treatment of pleural empyema, but these studies generally compared it with thoracotomy. The effectiveness of VATSD in the treatment of pleural empyema has been demonstrated in many recent studies. In 2001, Waller et al concluded that in addition to the benefits of the minimally invasive method (shorter hospital stay, less pain), VATSD was as effective as OD in the treatment of stage 3 empyema [19]. Chan et al retrospectively evaluated 77 patients who underwent surgery for empyema (41 VATSD and 36 OD) and reported that VATSD was as effective as OD and was superior in terms of length of hospital stay and postoperative pain [20]. Cardillo et al compared OD and VATSD in a retrospective study of 308 patients and concluded that thoracoscopic methods were the gold standard for empyema [21]. In their 2010 meta-analysis of 14 studies, Chambers et al reported that VATSD was superior to OD in the treatment of empyema in terms of postoperative pain, hospital stay, 30-day mortality, and complication and relapse rates [22].In another recent study, Samancılar et al performed VATSD in 54 patients and intrapleural fibrinolytic treatment in 24 patients and reported satisfactory results in both groups [23]. Pan et al also determined in their recent review that VATS was as good as thoracotomy for the treatment of empyema and was superior in terms of length of hospital stay and postoperative complications. They reported that thoracotomy might be required in a very small number of cases [8].
There are few articles on empyema surgery with U-VATS in the literature. In their 2010 study, Shahin et al evaluated 81 empyema cases, of which 60 underwent VATSD and 21 underwent OD. VATS was performed with 2 or 3 ports, but no comparison was made [24]. Bongiolatti et al evaluated 30 empyema patients who underwent U-VATS and 34 who underwent OD according to preoperative US findings and stated that VATSD was as effective as OD and was associated with a shorter length of hospital stay [25]. In another recent study, Ismail et al performed decortication by U-VATS to a total of 35 patients with stage 2 and 3 empyema. Their results regarding the length of hospital stay, chest tube duration, postoperative pain, and complications were consistent with the literature, and they also reported favorable cosmetic results [26].
In the present study, 3 patients had an early relapse, and 2 of those patients were treated by re-VATS while the other required OD. There was no late relapse, and the rate of minor complications was very low. Our results are consistent with the literature in terms of relapses and minor complications. The effectiveness of U-VATS and B-VATS were found to be consistent with the literature. As in other studies, U-VATS was associated with less postoperative drainage and pain than B-VATS. Although procedure times and postoperative hospital stays were shorter with U-VATS, the differences were not statistically significant.
Limitations of the Study
The retrospective design and inability to conduct multivariate analyses due to the small number of patients are limitations of this study. The main limitation is that our analysis was conducted in a small sample from a limited area.
In conclusion, VATS decortication is safe and effective for the treatment of stage 2 and 3 empyema in selected patients. Numerous studies already confirmed that VATS is indisputably superior to thoracotomy in terms of less postoperative pain, rapid recovery, and early discharge. The results of our study support the literature. The patients’ compliance with postoperative mobilization and respiratory exercises was nearly perfect due to the brief hospital stay and low pain, especially after U-VATS procedures. We believe that as surgeons gain more experience, U-VATS will become the gold standard in the treatment of empyema in the coming years.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Ethics approval
Approval for this retrospective, descriptive, single-center study was obtained from the Clinical Research Ethics Committee of Recep Tayyip Erdogan University Faculty of Medicine (decision no: 2020/193, dated 03.09.2020).
Authors’ contribution
KT; conceptualized and designed the study, collected and analyzed data. KT,HT; revised the final version of the manuscript and co-wrote the paper. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Reference
1) Godfrey MS, Bramley KT, Detterbeck F. Medical and Surgical Management of Empyema. Semin Respir Crit Care Med 2019; 40: 361-74.
2) Ferguson AD, Prescott RJ, Selkon JB, Watson D, Swinburn CR. The clinical course and management of thoracic empyema. QJM 1996; 89: 285-9.
3) Hamm H, Light RW. Parapneumonic effusion and empyema. Eur Respir J 1997; 10: 1150-6.
4) Falguera M, Carratalà J, Bielsa S, Garcia-Vidal C, Ruiz-Gonzales A, Chica I et al. Predictive factors, microbiology and outcome of patients with parapneumonic effusion. Eur Respir J 2011; 38: 1173-9.
5) Chalmers JD, Singanayagam A, Murray MP, Scally C, Fawzi A, Hill AT. Risk factors for complicated parapneumonic effusion and empyema on presentation to hospital with community-acquired pneumonia. Thorax 2009; 64: 592-7.
6) Higuchi M, Suzuki. Current status and prospect of medical and surgical management for thoracic empyema. Curr Challenges Thorac Surg 2020; 2: 39.
7) Davies HE, Davies RJO, Davies CWH. Management of pleural infection in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65: ii41–ii53.
8) Pan H, He J, Shen J, Jiang L, Liang W, He J. A meta-analysis of video-assisted thoracoscopic decortication versus open thoracotomy decortication for patients with empyema. J Thorac Dis 2017; 9: 2006-14.
9) Molnar TF. Current surgical treatment of thoracic empyema in adults. Eur J Cardiothorac Surg 2007; 32: 422-30.
10) Luh SP, Chou MC, Wang LS, Chen JY, Tsai TP. Video-assisted thoracoscopic surgery in the treatment of complicated parapneumonic effusions or empyemas: outcome of 234 patients. Chest 2005; 127: 1427-32.
11) Striffeler H, Gugger M, Im Hof V, Cerny A, Furrer M, Ris HB. Video-Assisted Thoracoscopic Surgery for Fibrinopurulent Pleural Empyema in 67 Patients. Ann Thorac Surg 1998; 65: 319-23.
12) Finley C, Clifton J, FitzGerald JM, Yee J. Empyema: An Increasing Concern in Canada. Can Respir J 2008; 15:85–89.
13) Akçay O, Acar T, Cantay S, Anar S. Minimally invasive approach to pneumothorax: Single port or two ports? Turkish J Thorac Cardiovasc Surg 2020; 28: 347-51.
14) Kutluk AC, Kocaturk CI, Akin H, Erdogan S, Bilen S, Karapinar K et al. Which is the Best Minimal Invasive Approach for the Treatment of Spontaneous Pneumothorax? Uniport, Two, or Three Ports: A Prospective Randomized Trail. Thorac Cardiovasc Surg 2018; 66: 589-94.
15) Cansever L, Sezen CB, Yaran OV, Bedirhan MA. Comparison of short-term quality of life in patients undergoing video-assisted thoracoscopic surgery versus thoracotomy. Turk Gogus Kalp Damar Cerrahisi Derg 2020; 28: 623-8.
16) Liu C, Liu L. Uniportal VATS: a sublimation of micro-invasive lung cancer resection. Zhongguo Fei Ai Za Zhi 2014; 17: 527-30.
17) DE LA Torre M, González-Rivas D, Fernández R, Delgado M, Fieira E, Méndez L. Uniportal VATS lobectomy. Minerva Chir 2016; 71: 46–60.
18) Shen KR, Bribriesco A, Crabtree T, Denlinger C, Eby J, Eiken P et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 2017; 153: e129-e146.
19) Waller DA, Rengarajan A. Thoracoscopic decortication: a role for video-assisted surgery in chronic postpneumonic pleural empyema. Ann Thorac Surg 2001; 71: 1813-6.
20) Chan DT, Sihoe AD, Chan S, Tsang DS, Fang B, Lee TW et al. Surgical treatment for empyema thoracis: is video-assisted thoracic surgery “better” than thoracotomy? Ann Thorac Surg 2007; 84: 225-31.
21) Cardillo G, Carleo F, Carbone L, Di Martino M, Salvadori L, Petrella L et al. Chronic postpneumonic pleural empyema: comparative merits of thoracoscopic versus open decortication. Eur J Cardiothorac Surg 2009; 36: 914-8.
22) Chambers A, Routledge T, Dunning J, Scarci M. Is video-assisted thoracoscopic surgical decortication superior to open surgery in the management of adults with primary empyema? Interact Cardiovasc Thorac Surg 2010; 11: 171-7.
23) Samancilar O, Akçam Tİ, Kaya SO, Ozturk O, Akcay O, Ceylan KC. The Efficacy of VATS and Intrapleural Fibrinolytic Therapy in Parapneumonic Empyema Treatment. Ann Thorac Cardiovasc Surg 2018; 24: 19–24.
24) Shahin Y, Duffy J, Beggs D, Black E, Majewski A. Surgical management of primary empyema of the pleural cavity: outcome of 81 patients. Interact Cardiovasc Thorac Surg 2010; 10: 565-7.



