

Summary
Background: The cytological examination of pleural effusions is a quick and effective diagnostic method used to elucidate the etiology. The aim of this study is to reveal cytopathological diagnosis distributions by retrospectively examining seven-year pleural cytology and analyze cyto-histopathological correlations in cases with biopsy in this study.Materials and Methods: In this study, 545 pleural fluid aspiration materials that resulted as Bolu Abant İzzet Baysal University İzzet Baysal Training and Research Hospital between 2014 and 2020 were evaluated retrospectively. The evaluation results were divided into four groups as inconclusive, negative for malignancy, suspected malignancy, and malignant cytology. The biopsy diagnoses of 64 cases were grouped as inadequate, benign, and malignant. Sensitivity and specificity values were calculated by looking at the relationship between cytology and biopsy diagnoses.
Results: According to the cytology diagnoses, 435 cases (79.8%) were negative for malignancy, 43 cases (7.9%) were malignant, 32 cases (5.9%) were suspected of malignancy, and 35 cases (6.4%) were inconclusive. When the diagnoses of the cases with both cytology and biopsy analyses were compared, the sensitivity of the cytology was 96.7%, the specificity was 91.2%, the positive predictive value was 90.6%, and the negative predictive value was 96.7%. The biopsy and cytology results were found to be compatible (p < 0.05)
Conclusions: Pleural effusion develops due to different etiologies, and the cytological examination of pleural fluid is an important diagnostic method for malignancy. In our region, the most common cause of pleural effusion is benign pathologies which are consistent with data in the literature. The correlations between cytology- and biopsy-based diagnosis results were found high.
Introduction
The pleura is the serous membrane that surrounds the lungs and thoracic cavity, consisting of two layers as parietal and visceral layers [1,2]. Pleural fluid is produced by the parietal pleura and reabsorbed by lymphatic channels [3]. A disruption in this homeostasis causes pleural effusion [3].Pleural effusion occurs with the accumulation of fluid in the pleural cavity in intrathoracic or systemic diseases [1,4]. Fluid may accumulate in the pleural cavity because of benign or malignant causes [5,6]. Biochemical, microbiological, and cytological examinations are performed in pleural fluids taken by thoracentesis in patients with pleural effusion [7]. Pleural cytology examination also provides useful information in terms of diagnosis, tumor staging, and guiding treatment, protecting patients from a more invasive examination and reducing health expenditures [7,8]. When pleural cytology is not diagnostic, pleural biopsy or thoracoscopic biopsy is performed [7].
The sensitivity of pleural cytology varies by 40-87% because of the retrospective nature of studies, differences in the criteria examined and cytopathological methods used in different studies, and the usage of old guidelines [7,8]. It is known that cytology has a high diagnostic value in malignant pleural fluids [9,10]. It has been reported that the most common causes of malignant pleural effusion are lung cancer and breast cancer [1].
The aim of this study is to examine pleural cytology samples sent to the pathology laboratory because of pleural effusion, reveal the characteristics and cytopathological diagnosis distributions of seven-year pleural cytology results retrospectively, and analyze cyto-histopathological correlations in cases with biopsy.
Methods
This study is a retrospective descriptive study. In this study, 545 pleural fluid aspiration materials belonging to cases who underwent thoracentesis because of pleural effusion with results sent to Bolu Abant İzzet Baysal University, İzzet Baysal Training and Research Hospital between 2014 and 2020 were evaluated retrospectively. The clinical parameters, cytology results, and biopsy results of the cases were obtained from archive records. Our study was conducted in accordance with the Declaration of Helsinki and was approved by the Bolu Abant İzzet Baysal University Ethics Committee for Clinical Research (2021/39).In cases with benign pleural cytology, one of the cytology samples was evaluated, and the other cytology samples were not included in the study. In the case of multiple pleural cytology results, malignant cytology results were included in the study. The cytology samples were stained with the Papanicolaou method and the May Grunwald-Giemsa stain. In cases where a cell block could be obtained, cell block was created and stained with hematoxylin and eosin. The cytopathological evaluation results were divided into four groups as inconclusive, negative for malignancy, suspected malignancy, and malignant cytology [11,12]. Malignant pleural effusion was accepted as the presence of malignant cells in cytology [13].
In 64 cases with biopsy results, the biopsy results were classified as inconclusive, benign, and malignant. The statistical comparisons and cyto-histopathological correlation analyses were carried out in terms of the cytology and biopsy diagnoses.
Statistical Analyses
In this study, frequency analysis and percentage distribution analyses of the data were carried out using the SPSS 21 software (Version 21, Armonk, NY: IBM Corp). The variables did not show normal distribution, and they are presented with median (minimum–maximum) values. The comparative distributions results are given with cross-tables. Sensitivity and specificity values were calculated by looking at the compatibility between the cytology and biopsy diagnoses. P < 0.05 accepted as statistically significant.
Results
In this study, the ages of the patients with 545 pleural cytology results ranged from 2 to 105, with a mean age of 66.3. Three hundred and twenty-nine of the cases were male (60.4%), and 216 (39.6%) were female.According to their cytological diagnoses, 435 cases (79.8%) were negative for malignancy, 43 cases (7.9%) were malignant, 32 cases (5.9%) were suspected of malignancy, and 35 cases (6.4%) were inconclusive. When the suspicion of malignancy in the cytological diagnoses was evaluated together with the malignant group, 75 cases were diagnosed as malignant, and their mean age was 65.2 years. The characteristics and diagnostic distributions of the pleural cytology examinations are given in table 1.
Table 1: Characteristics and diagnostic distribution of pleural cytology.
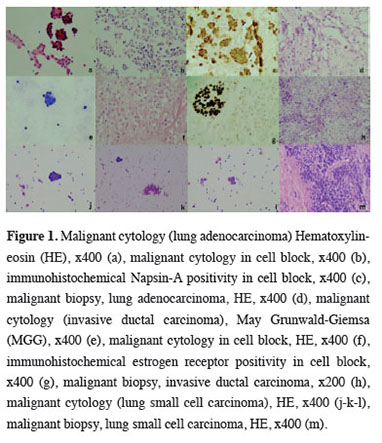
A cell block could not be obtained in 371 cases (68.1%), and cell blocks were present in 174 cases (31.9%). Immunohistochemical staining was applied to the cell blocks for diagnosis in 40 cases (7.3%), and the most frequently used immunohistochemical stains were TTF-1, CK7, and Napsin-A. There were 32 cases which showed specific malignant diagnoses cytologically. The most frequently identified diagnoses of these cases were lung adenocarcinoma (14 cases, 43.8%) and breast invasive ductal carcinoma (5 cases, 15.6%) (Figure 1). The distribution of cytological diagnoses by sex is given in table 2.
 Click Here to Zoom |
Figure 1: Malignant cytology (lung adenocarcinoma) Hematoxylin-eosin (HE), x400 (a), malignant cytology in cell block, x400 (b), immunohistochemical Napsin-A positivity in cell block, x400 (c), malignant biopsy, lung adenocarcinoma, HE, x400 (d), malignant cytology (invasive ductal carcinoma), May Grunwald-Giemsa (MGG), x400 (e), malignant cytology in cell block, HE, x400 (f), immunohistochemical estrogen receptor positivity in cell block, x400 (g), malignant biopsy, invasive ductal carcinoma, x200 (h), malignant cytology (lung small cell carcinoma), HE, x400 (j-k-l), malignant biopsy, lung small cell carcinoma, HE, x400 (m). |
Table 2: Distribution of cytological diagnoses by gender.
The biopsies of 64 cases (12.5%) were also available from pleural cytology examinations. The biopsies were taken from the pleura (40 cases, 62.5%), lung (15 cases, 23.4%), peritoneum (3 cases, 4.7%), colon (2 cases, 3.1%), lymph nodes, endometrium, liver, and stomach (1 case in each, 1.6%). In the biopsies, 34 cases (53.1%) were diagnosed as malignant, 22 cases (34.4%) were diagnosed as benign, and 8 cases (12.5%) were diagnosed as inconclusive. The most common malignant biopsy diagnoses were lung adenocarcinoma cases (13 cases, 20.3%), and the second most common were lung squamous cell carcinoma cases (5 cases, 7.8%). Immunohistochemical staining was applied to 36 of the biopsy materials (56.3%). The most frequently used immunohistochemical stains were TTF-1, which is an adenocarcinoma marker, Napsin-A, and calretinin and WT-1, which are mesothelioma markers. The diagnostic distributions of the biopsies are given in table 3.
Among the cases with malignant biopsy-based diagnoses, 91.2% were diagnosed as malignant cytology results. However, there were 3 cases with malignant biopsy diagnoses and negative cytology results for malignancy. The biopsy diagnoses of these cases were lung adenocarcinoma, lung squamous cell carcinoma, and endometrioid adenocarcinoma.
Of the cases with biopsy results that were benign, 95.5% had benign cytology results. The cytology of 85% of the cases with a biopsy-based diagnosis of chronic pleuritis was negative for malignancy, and one case was diagnosed as malignant in cytology. This case was diagnosed as lung adenocarcinoma in cytology, but chronic pleuritis was diagnosed in pleural biopsy in which a lung biopsy was not taken. The diagnoses of 87.5% of the materials with inconclusive biopsy results were negative in terms of malignancy, and there was no malignant biopsy in this group. The cytology of 92.3% of the cases diagnosed as lung adenocarcinoma was malignant. Malignant cytology was present in 80% of the cases diagnosed with lung squamous cell carcinoma. The mean age of the patients among the malignant biopsy results was 61.5 years.
When the cytological and biopsy diagnoses were compared, the sensitivity value of the cytology was 96.7%, the specificity value was 91.2%, the positive predictive value was 90.6%, and the negative predictive value was 96.7%. The diagnostic value of the cytological examinations is given in table 4. The biopsy and cytology results were found to be compatible (p < 0.05).
Discussion
The etiologies of pleural effusion may vary according to the age of the patient, geographical region, society, hospital, and clinic [2,14,15,16]. The pleura may be involved with primary and secondary tumors [17]. In malignancy, increased cellular permeability, tumor-associated angiogenesis, increased pleural fluid production, and lymphatic obstruction are the causes of pleural effusion in pleural metastasis [5,6,17]. The local and systemic effects of the tumor, treatment complications, and the direct involvement of the pleura with malignant cells are fluid formation mechanisms [6,17].In malignant cases, cytopathological evaluation is more valuable than pleural tissue biopsy [1]. Cytological examination has advantages such as the easy acquirement of materials, fast results, fewer complications, and inexpensiveness [11]. The cytological diagnostic value in malignant pleural effusions varies in the range of 9-80%, and the diagnostic value in pleural biopsy varies in the range of 11-70% [2]. Factors such as cancer type, the experience level of the cytologist, and the volume of pleural fluid affect changes in these rates [18].
It was stated that at least 50-75 mL of aspiration material should be sent to increase the efficiency of the analysis in pleural cytology [3]. The cell block method used after the centrifugation of the pleural fluid is a method that supports the diagnosis because it reveals the tissue structure and allows immunohistochemical and molecular analyses [7,10,17]. In our study, the cell block method was performed in 26 (60.5%) of the cases with malignant cytology results.
When a malignant pleural disease is confirmed, two positive mesothelial markers (among calretinin, CK5/6, Wilms tumor 1, D2-40) and at least two negative immunohistochemical lung adenocarcinoma markers (TTF-1, CEA, Ber-EP4) to distinguish malignant pleural mesothelioma from secondary tumors contribute to the differential diagnosis [3,10]. In our study, immunohistochemical staining was applied to the cell blocks, and the most commonly used markers were TTF-1, Napsin-A, calretinin, and WT-1.
Lung cancer, breast cancer, and lymphoma constitute approximately 75% of malignant pleural effusions [4,5]. In our study, the most common cause of malignant pleural effusion was lung adenocarcinoma, and the second most common cause was breast invasive ductal carcinoma.
Primary cancer foci may not be detected in malignant pleural effusions [1]. If the cytology result is negative, pleural biopsy is necessary [17]. However, it may show lower sensitivity in the case of insufficient tissue sampling and focal metastasis to the pleura [7]. In our study, there was a case with lung adenocarcinoma in cytology and a benign pleural biopsy. Since lung biopsy was not performed in this case, the biopsy result was negative.
The cytology experience of Ozdamar et al with 9043 cases showed that pleural cytology constituted 3% of all cytology results [11]. Cobanoglu found the most common cases as lung squamous cell carcinoma (9 patients, 30.60%) in their study of 49 malignant effusion cases [6].
In the study by Assawasaksakul et al, malignant diagnoses were made in 278 (78.8%) of 353 pleural cytology materials [7]. One hundred and thirty-two patients were diagnosed with lung non-small-cell cancer, 33 patients were diagnosed with breast cancer, and 31 patients were diagnosed with hematological malignancies [7]. In the study of 3077 pleural effusions conducted by Porcel et al, 840 patients (27%) had a malignant etiology [16]. Of the malignant cytology results, 309 (37%) consisted of lung malignancies, 123 (16%) consisted of breast malignancies, and 82 (10%) consisted of unknown primary malignancies [16]. Adenocarcinoma was commonly present in lung the lung malignancies (150 cases) [16].
In the study by Bayrak et al, malignant causes were found to be the largest group with 56 (36.6%) patients in 153 cases with pleural effusion [4]. Among the malignant effusion cases, the most common cause was lung cancer with 30 (54%) cases, and the second most common cause of malignant effusion was breast cancer with 9 (34.6%) patients [4]. In the distributions of the lung cancer cases according to histological types, there were 9 (30%) non-small-cell lung cancers, 10 (34%) squamous cell carcinomas, 5 (16%) adenocarcinomas, and 6 (20%) small-cell lung cancers [4].
Dagli et al reported 3 (1%) inconclusive cases, 8 (2.7%) atypical cases, 246 (82.6%) benign cases, 10 (3.4%) suspicious cases, and 31 (10%, 4) malignant cases [1]. They reported that the cause of malignant pleural effusion was metastatic carcinoma in 24 (8.1%) cases and malignant mesothelioma in 7 (2.3%) cases [1]. In the study by Gonlugur et al with 454 cases, malignant pleural effusion was seen in 32.6% of the cases, and the most common pleural effusions were malignant mesothelioma cases due to environmental asbestos exposure (67 cases, 14.8%) [14]. Uzunlar et al found malignant pleurisy in 54 (33.54%) cases and benign pleurisy in 107 (66.45%) cases [9]. In the malignant pleural effusion cases, the diagnostic value of cytology was found to be 55.56%, and the diagnostic value of pleural biopsy was 59.25% [9].
In the study by Dumanlı et al, the cytological malignant effusion rate was 17.15% (6 patients), the malignant pleural biopsy rate was 25.71% (9 patients), and the most common malignant tumors diagnosed in biopsy were adenocarcinoma metastasis at 8.57% (3 patients) and malignant mesothelioma at 5.71% (2 patients) [5]. Since our study was not conducted in an endemic region, there was no case of mesothelioma.
Thomas et al mentioned right sided pleural effusion as the most common case. The most common cause was reported as tuberculosis (344 cases, 84.5%) with malignant effusion at a rate of 5.2% in 21 patients [19]. The most common malignancies were lung adenocarcinoma (8 patients, 38%), breast carcinoma, and lymphoma (4 patients each, 19%) [19]. Riquet et al sampled pleural fluid during lung resection in 143 patients [12]. Pleural fluid positivity was observed in 10.7% (13/121) of the cases diagnosed as malignant in biopsy [12]. Positive adenocarcinomas were observed more frequently [12]. Mercer et al diagnosed 325 (32.6%) cases as malignant pleural effusion from 998 pleural cytology materials [13]. The most common malignant diagnoses were breast cancers, non-small-cell lung carcinoma, and gynecological cancers, respectively [13].
In our study, the most common cause of pleural effusion was benign etiologies in accordance with the literature data. The mean age in the malignant effusion cases was 65.2. In our study, 2 patients (6.3%) were diagnosed with malignant lymphoma.
In the study by Tokgoz et al with 240 cases, 76 (32%) of the cases were female, 164 (68%) were male, and the mean age of all cases was 58 ± 20 years [15]. Among the effusion cases, 138 (57%) were on the right side, 71 (30%) were in the left hemithorax, and 31 (13%) were bilateral [15]. Malignant effusion constituted 25.4% of the cases [15]. The most common diagnoses were 38 (62%) primary lung cancer cases, 8 (13%) malignant mesothelioma cases, and 4 (7%) lymphoma-induced effusion cases [15]. In their study of 126 unilateral pleural effusion cases, Bintcliffe et al reported a mean age of 75 years, with 83 (66%) male cases and 43 (34%) female cases [20]. Right pleural effusion was seen in 78 cases (62%), and left pleural effusion was seen in 48 cases (38%) [20]. Malignant pleural effusion was diagnosed in 58 patients (46%) [20]. The most common types of malignancies were malignant mesothelioma (18 patients, 31%), lung cancer (17 patients, 29%), and breast cancer (5 patients, 9%) [20]. Similar to these studies, effusion was observed mostly in the male patients and in the right pleura in our study. Our malignant effusion rate in cytology was 14%.
In the study by Arnold et al which is the largest prospective study of 921 patients, a mean age of 70.2 ± 13.8 years was found with male predominance [8]. They performed cell block analyses in cases where 40 mL of cytology samples were obtained from the patients [8]. They found the malignant effusion rate to be 56% in 515 patients. Of these cases, the most common ones were lung cancer cases (32%, 166 cases, adenocarcinoma most common with 100 cases), which were followed by mesothelioma cases (29%, 148 cases) [8]. In their study, the mean sensitivity was 46%, and they stated that cytology was more valuable in detecting adenocarcinomas [8]. In our study, lung adenocarcinoma was the most common tumor, and a high consistency was found with a kappa value of 0.875 in our cytology and biopsy diagnoses.
Since this study is a retrospective study, there were parts for which sufficient information could not be obtained. The study focused on malignant effusion cases, and benign etiologies could not be typified. In the study, the inability to send a sufficient amount of fluid to form a cell block limited immunohistochemical staining. Additionally, the limitations of the study were the inability to diagnose the cases whose cytological material came to our laboratory with biopsies performed in another center.
In conclusion, pleural effusion develops due to different etiological reasons, and the cytological examination of pleural fluid is an important diagnostic method for malignancy. In our region, the most common cause of pleural effusion is benign pathologies which are consistent with data in the literature. The most common cause of malignant pleural effusion is lung adenocarcinoma. The correlations between the cytology and biopsy diagnoses were found high. Since malignant pleural effusions often develop secondary to lung adenocarcinomas, it is thought that the lung should be investigated first for the primary focus in pleural effusions.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Ethics approval
The study was approved by Bolu Abant İzzet Baysal University Ethic Committee for Clinical Research (2021/39).
Authors’ contributions
SED,SO: involved in conceptualization, design, data analysis, manuscript writing, and review. SO: participated in data collection, data entry, and data analysis. SED,SO: participated in data analysis, manuscript writing, and review. All authors read and approved the final manuscript.
Reference
1) Dagli AF, Kucuk S, Sezer M, Ucer O. Cytopathologic diagnosis in pleural effusion and cyto-histopathologic correlation. Turkish J Pathol 2011; 27: 12.
2) Yanik S, Turgut O, Ay AA, Durkaya S, Çam A, Karaca T et al. Retrospective analysis of pleural fluid cytology. Kırıkkale Uni Med J 2014; 16: 1-4.
3) Sundaralingam A, Bedawi EO. Diagnostics in Pleural Disease. Diagnostics 2020; 10: 1-20.
4) Bayrak MG, Erkan L, Uzun O, Findik S, Atıcı AG, Özkaya S. Evaluation of 153 patients with pleural effusion. Respir Dis 2006; 17: 66-72.
5) Dumanlı A, Oz G. VATS plevra biopsy experiences: analysis of 35 cases. Med J SDU 2020; 27: 261-6.
6) Çobanoglu U. Clinical approach in malignant pleural effusion: analysis of 49 patients. Van Med J 2007; 14: 74-9.
7) Assawasaksakul T, Boonsarngsuk V, Incharoen P. Comparative study of conventional cytology and cell block method in the diagnosis of pleural effusion. J Thorac Dis 2017; 9: 3161-7.
8) Arnold DT, Fonseka D De, Perry S, Morley A, Harvey JE, Medford A et al. Investigating unilateral pleural effusions: The role of cytology. Eur Respir J 2018; 52: 1-9.
9) Uzunlar A, Buyukbayram H, Kırbas G, Yaldız M, Yılmaz F, Arslan A. Diagnostic value of pleural fluid cytology and biopsy (A retrospective study). Respir Dis 2015; 11: 169-73.
10) Porcel JM. Malignant pleural effusions because of lung cancer. Curr Opin Pulm Med 2016; 22: 356-61.
11) Ozdamar SO, Bektas S, Barut F, Bahadir B, Numanoglu G, Gun BD et al. Cytology experience of Zonguldak Karaelmas University Medical Faculty Hospital between 2003-2005 years. Turkish J Pathol 2006; 22: 92-5.
12) Riquet M, Badoual C, Barthes FLP, Lhote FM, Souilamas R, Hubsch JP et al. Visceral pleura invasion and pleural lavage tumor cytology by lung cancer: A prospective appraisal. Ann Thorac Surg 2003; 75: 353-5.
13) Mercer RM, Varatharajah R, Shepherd G, Lu Q, Castro-Añón O, McCracken DJ et al. Critical analysis of the utility of initial pleural aspiration in the diagnosis and management of suspected malignant pleural effusion. BMJ Open Respir Res 2020; 7: 1-7.
14) Gönlügür T, Gönlügür U. Retrospective analysis of 454 pleural effusions. Inonu Unv Med J 2007; 14: 21-5.
15) Tokgoz F, Goksenoglu N, Bodur Y, Aksoy E, Aktas O, Sevim T. Retrospective analysis of 240 cases with pleural effusion. Eurasian J Pulmonol 2014; 16: 78-83.
16) Porcel JM, Esquerda A, Vives M, Bielsa S. Etiology of pleural effusions: analysis of more than 3,000 consecutive thoracenteses. Arch Bronconeumol. 2014; 50: 161-5.
17) Lombardi G, Zustovich F, Nicoletto MO, Donach M, Artioli G, Pastorelli D. Diagnosis and treatment of malignant pleural effusion: A systematic literature review and new approaches. Am J Clin Oncol Cancer Clin Trials 2010; 33: 420-3.
18) Porcel JM. Diagnosis and characterization of malignant effusions through pleural fluid cytological examination. Curr Opin Pulm Med 2019; 25: 362-8.



