

2Department of Radiology, Başakşehir Çam and Sakura City Hospital, İstanbul, Turkey DOI : 10.26663/cts.2023.003
Summary
Background: The study aimed to examine the prevalence and radiological features of Elastofibroma Dorsi (ED), detected incidentally in patients who underwent CT for various reasons.Materials and Methods: A total of 8378 chest CT examinations were reviewed retrospectively for ED from January 2019 to April 2020. Lesion side, thickness, density, and whether stated in the initial report were examined.
Results: Of the 8378 patients, 87 patients (1.03%) (41 women and 46 men) had a total of 114 ED. There was no significant difference in the mean age of ED patients regarding gender (p < 0.001). When the ages of the patients included in the study with (n = 87, 52.0 ± 14.7) and without ED (n = 8291, 42.0 ± 15.2) were compared, there was a statistically significant difference (p < 0.001). The rate of bilateral ED was 31%. A positive correlation was determined between mean ED thickness and age (p = 0.001 and p = 0.041 for right and left, respectively). It was noted that none of the patients were diagnosed in the first radiological report.
Conclusions: ED is a slow-growing non-neoplastic, soft tissue tumor with a typical periscapular location. Being aware of this entity will prevent the need for advanced diagnostic evaluation. The characteristic appearance of these lesions will reduce unnecessary biopsy and surgery with increased radiologist awareness of their recognition.
Introduction
Elastofibroma Dorsi (ED) is a non-neoplastic, slow-growing, benign, rare soft tissue tumor [1]. It is generally observed between the fourth and seventh decades of life, mainly in women [2,3]. 12-73% of cases are bilateral [4-6]. Although the exact etiology remains unclear, friction, trauma, tissue degeneration, and genetic predisposition among co-occurrence hypotheses have been described in the literature [6].Clinically, 50% of cases are asymptomatic, and when symptoms occur, mild or severe pain accompanied by a clicking sound during abduction and adduction of the shoulder is typical for ED [7,8,9].
In the cross-sectional examination, ED contours may be sharp or ambiguous. The mass contains both fibrous tissue and adipose tissue. CT and MRI are particularly effective in diagnosis as they demonstrate the layered nature of the tumor’s fat and fibrous tissue (with imaging properties similar to muscle on CT and MRI). Imaging features and localization are usually typical, and a definitive diagnosis can be made in most cases. CT is less sensitive in demonstrating adipose tissue than MRI, and therefore, it can be determined as a homogeneous mass slightly lower than muscle density on CT [9].
This study aimed to examine the prevalence and radiological features of ED, detected incidentally in a large population of patients who had CT for various reasons.
Methods
Patient cohortAll patients referred to the Radiology Department for various reasons (e.g., nodule follow-up, infection, lung cancer, staging of other tumors, acute trauma, mediastinal mass) between January 2019 and April 2020 were evaluated retrospectively. A total of 8378 chest CTs constituted the study group. Of these patients, 4584 (55.3%) were male, 3704 (44.7%) were female, and the mean age was 42.1 ± 15.2 years (0-94). Approval from the Institutional Review Board of Haseki Research and Traning Hospital was obtained without the requirement for patient informed consent because of the retrospective origin of the study (Decision number: 2020-131).
Imaging
All CT images were acquired using a 128 detector CT alignment (Philips Ingenuity, Netherlands). Images were obtained with the patient at the end of inspiration and supine position with 120 kV tube voltage, tube current-exposure time product, 512x512 matrix, 415 mm FOV, 200-300 mAs, and 1.25 mm section thickness after reconstruction.
Image analysis
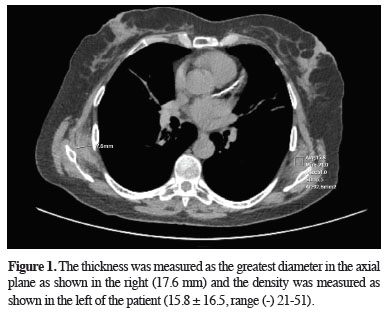
After reviewing all patients retrospectively, the two radiologists evaluated the determined ED lesions with consensus regarding lesion density and thickness, and the lesion side was noted. While evaluating the lesion size, the thickness was measured as the greatest diameter in the axial plane (Figure 1). The initial radiology report of the patients with ED was reviewed regarding whether the lesion was mentioned in the first evaluation.
 Click Here to Zoom |
Figure 1: The thickness was measured as the greatest diameter in the axial plane as shown in the right (17.6 mm) and the density was measured as shown in the left of the patient (15.8 ± 16.5, range (-) 21-51). |
Statistical Analysis
SPSS 15.0 for Windows program was used for statistical analysis. Descriptive statistics were presented as numbers and percentages for categorical variables and mean, standard deviation, minimum, maximum, median, and interquartile range values for numerical variables. Numerical variables in two independent groups were compared using the Student’s t-test when the normal distribution condition was met and the Mann-Whitney U test when the normal distribution condition was not met. Chi-Square Test performed the comparisons of the rates in the groups. Correlations were analyzed by Spearman Correlation Analysis when the parametric test condition was not met. The statistical alpha significance level was accepted as p < 0.05.
Results
In our study, 87 (1.03%) of 8378 patients had a total of 114 EDs. There were unilateral lesions in 60 (69.0%) patients and bilateral (31.0%) lesions in 27 (Figures 2,3). Of the ED patients, 46 (52.9%) were male, and 41 (47.1%) were female. The mean age was 54.6 ± 14.8 years (range 20-85) for females and 49.7 ± 14.5 years (range 24-81) for males. There was no significant difference in the mean age of men and women with ED (p < 0.001) (Table 1). When the ages of the patients included in the study (n = 87, 52.0 ± 14.7) with and without ED (n = 8,291, 42.0 ± 15.2) were compared, there was a statistically significant difference (p < 0.001) (Table 2).Table 1: The entire patient population; Number of patients with and without ED, age, and gender.
 Click Here to Zoom |
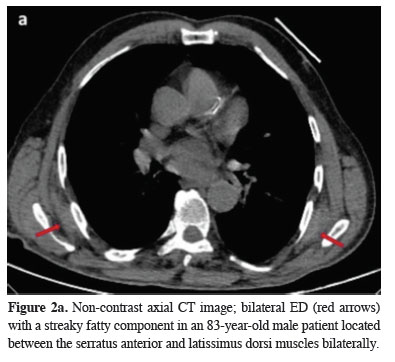
Figure 2a: Non-contrast axial CT image; bilateral ED (red arrows) with a streaky fatty component in an 83-year-old male patient located between the serratus anterior and latissimus dorsi muscles bilaterally. |
 Click Here to Zoom |
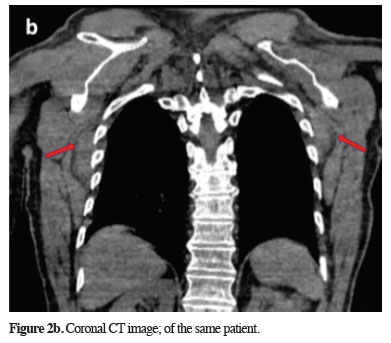
Figure 2b: Coronal CT image; of the same patient. |
 Click Here to Zoom |
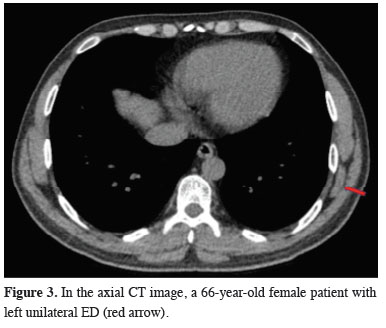
Figure 3: In the axial CT image, a 66-year-old female patient with left unilateral ED (red arrow). |
Table 2: Average age of patients with and without ED.
In our study, the rate of bilateral ED was 31%. There was no significant difference between the mean age of patients with bilateral lesions and those with unilateral lesions (p = 0.101), and no significant gender difference was determined in both patient groups (p = 0.737) (Table 3).
Table 3: Age and gender of patients with unilateral and bilateral ED.
All lesions were evaluated separately, and the mean ED thickness was measured as 8.2 ± 6.5 mm (range 2.9-54.2). It was measured as 8.95 ± 5.81 mm (3-27) on the right and 7.60 ± 6.89 mm (2-54) on the left side. A positive correlation was determined between mean ED thickness and age (p = 0.001 and p = 0.041 for right and left, respectively). There was no significant difference in mean ED thickness regarding the side and gender (p = 0.086 and p = 0.621, respectively).
The mean ED density was 18.4 ± 24.3 (-58-61.5) HU. It was 14.58 ± 27.70 (-58.6-61.5) HU on the right, and 21.28 ± 21.15 (-32-57.7) HU on the left side, with no significant difference between them (p = 0.243). When the right and left side densities were compared according to the genders, no significant difference was determined (p = 0.947 and p = 0.792 for the right and left, respectively). In patients with ED, there was no significant correlation between density and size for both sides (p = 0.172 and p = 0.352 for right and left, respectively). The left-sided ED frequency was significantly higher than the right-sided ED (p = 0.048).
Almost all ED patients had lesions isodense with adjacent muscle structures and contained linear hypodense areas on imaging. When the initial imaging reports of the patients were reviewed, none of the lesions were mentioned in the first radiological report. All patients with ED had non-contrast imaging examinations. Therefore, the enhancement feature could not be evaluated. When evaluated from the clinical point of view by retrospectively reviewing the hospital electronic medical record system, none of the patients with ED had dorsalgia or shoulder pain, which are the main symptoms of this entity.
Discussion
ED is a benign, rare soft tissue tumor with a characteristic appearance and localization on CT and was first defined by Jarvi and Saxen in 1961 [10,11]. The typical location is at the level of the lower end of the scapula between the latissimus dorsi and serratus anterior muscles. Elastofibroma develops in this localization in 99% of cases [4]. However, different localizations, including the olecranon, tricuspid valve, interspinal, ligamentum flavum, foot, greater trochanter, deltoid, axilla, intra-articular, intraspinal and ischial tuberosity, stomach, cornea, and mediastinum, have been reported in the literature [12-21]. Nagamine et al diagnosed elastofibroma in the olecranon and ischial tuberosity in addition to the subscapular level, which is classically located in the patient group, and associated this condition with genetic and structural factors [4]. In elastofibromas observed outside the classical localization described in the literature, the diagnosis was made almost entirely pathologically.A retrospective study by Brandser et al, in which they evaluated 268 patients describing the prevalence and imaging features of ED in 1998, indicated the prevalence of ED as 2% in the group of patients over 60 years of age. Our study determined the prevalence of ED as 1.03%. We associated this result with the lower mean age (42.1 ± 15.2) of our entire study population compared to the aforementioned study [20,22]. In the study of Tepe et al, the prevalence of ED was 2.73%, and the mean age of patients with and without ED was 68.2 and 56.1 years, respectively [12]. In our study, this result was 42.0 and 52.0, respectively, and similarly, the mean age of our patients without ED population was low. In their study involving the Jarvi and Lansimies autopsy series, they reported the prevalence of ED as 16%. This difference with the CT series can be attributed to the small size of the lesions in the second study and the macroscopic definition of some lesions as only a streak in the fascia [23].
In studies with large series in which ED was reported in the literature, the female gender was generally more common [6,12,22-25]. Although there was no statistically significant difference in our study, there were 46 male patients (52.9%) in total with predominance. In the study of Chandrasekar et al, similar to our study, there was male predominance with the rate of 80% [26]. The tendency of ED to be bilateral has been detected in many studies, and it has been reported at a rate of approximately 12-73% [4-6,27-29]. In our study, the rate of bilateral lesions was 31%. There was no significant difference between the mean age of patients with bilateral lesions and those with unilateral lesions, and our result was consistent with the literature [12].
A significant relationship was observed between ED thickness and age on both sides in our study. In the literature, a similar relationship was reported by Tepe et al only for the right side [12]. However, there was no significant difference in ED thicknesses when compared regarding the side or gender in our study. Moreover, the mean density of ED was measured as 18.4 ± 24.3 HU on the right and 21.28 ± 21.15 HU on the left side. Negative HU values of fat density depend on the fat component in the tumor, and according to our knowledge, such a measurement has not been evaluated before in the literature. None of the patients with ED in our study were diagnosed with ED at the initial report. Although the diagnosis of ED is easily made with CT, it can be overlooked in the first report, similar to other studies in the literature [12,22,31].
Diagnosis of ED is made radiologically by MRI and CT according to the typical location of the tumor and imaging characteristics [7,11,22,30]. Lesions with a fibrous component and linear adipose tissue in typical periscapular localization, especially when bilateral, can be diagnosed as ED accurately without biopsy. Adipose tissue has a hypodense appearance on CT and T1-T2 hyperintense signal on MR. The imaging feature of fibrous tissue is similar to muscle tissue in CT and MRI [12]. The sensitivity of CT in the diagnosis of ED is lower than MRI. After intravenous gadolinium, the mass shows minimal-moderate or significant contrast material uptake [3].
ED excision is not recommended in asymptomatic cases since no malignant transformation has been described before [6,7,31]. When the biopsy is required, open biopsy or at least core needle biopsy is recommended. Fine needle biopsy is not recommended due to the hypocellular nature of the tumor [7]. Tumors that may be located in this region in the differential diagnosis include fibroma (in cases with fibrous predominance), aggressive fibromatosis, metastasis, hemangioma, and atypical lipoma and liposarcoma in patients with lipomatous component [7,33-36]. These tumors can be distinguished from ED radiologically by CT and MRI as they do not demonstrate the laminar pattern of ED.
Microtrauma, elastin degeneration, enzyme defect, genetic factors, and vascular insufficiency have been suggested in the etiology [36-41]. More than 50% of ED cases are asymptomatic. However, some patients may experience swelling, shoulder stiffness, friction noise with shoulder movements, and pain [34,42,43]. Rarely, clinical findings may change, and ED may be mistakenly confused with subacromial bursitis or rotator cuff tear [27].
The treatment of ED is surgical excision. A surgical operation is recommended when the tumor is greater than 5 cm, the patient is symptomatic and radiological findings are compatible with malignancy [44]. A conservative approach can be preferred if the patient is asymptomatic, and the tumor can be followed up clinically. Radiotherapy can be used in high-risk patients for surgery [14].
None of the patients with ED had either dorsalgia or shoulder pain, which are the main symptoms of ED. The condition might be related to the small mean size of ED and only six patients had a tumor size of greater than 2 cm. Patients diagnosed with ED are under follow up by thoracic surgery clinic in our hospital but there are no patients requiring surgery yet.
The main limitation of our study was the lack of histopathological verifications of the patients.
In conclusion, ED is a slow-growing, non-neoplastic soft tissue tumor with a typical periscapular location. Being aware of this entity and its radiologic features will prevent the need for advanced diagnostic evaluation. The characteristic appearance of these lesions, typically bilateral, will reduce unnecessary biopsy and surgery with increased radiologist awareness of their recognition.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Ethics approval
Approval from the Institutional Review Board of Haseki Research and Traning Hospital was obtained without the requirement for patient informed consent because of the retrospective origin of the study (Decision number: 2020-131).
Authors’ contribution
BKY,SÖ,Tİ,TSC,NG,BA; material preparation, data collection and analysis. BKY; the first draft of the manuscript was written by. BKY, RT: revised the final version of the manuscript. All authors read and approved the final manuscript. All authors contributed to the study concep¬tion and design.
Reference
1) Kaplanoğlu H, Turan A, Kaplanoğlu V, Uçar, Selek Y. Bilateral elastofibroma dorsi: olgu sunumu. CausaPedia 2020; 9: 20-3.
2) Kara M, Dikmen E, Kara SA, Atasoy P. Bilateral elastofibroma dorsi: proper positioning for an accurate diagnosis. Eur J Cardiothorac Surg 2002; 22: 839-41.
3) Schick S, Zembsch A, Gahleitner A, Wanderbaldinger P, Amann G, Breitenseher M et al. Atypical appearance of elastofibroma dorsi on MRI: case reports and review of the literature. J Comput Assist Tomogr 2000; 24: 288-92.
4) Nagamine N, Nohara Y, Ito E. Elastofibroma in Okinawa. A clinicopathologic study of 170 cases. Cancer 1982; 50: 1794-805.
5) Pilge H, Hesper T, Holzapfel BM, Prodinger PM, Straub M, Krauspe R. Elastofibroma: clinical results after resection of a rare tumor entity. Orthop Rev (Pavia) 2014; 6: 80-4.
6) Deveci MA, Ozbarlas HS, Erdogan KE, Bicer OS, Tekin M, Ozkan C. Elastofibroma dorsi: Clinical evaluation of 61 cases and review of the literature. Acta Orthop Traumatol Turc 2017; 51:7-11.
7) Daigeler A, Vogt PM, Busch K, Pennekamp W, Weyhe D, Lehnhardt M et al. Elastofibroma dorsi–differential diagnosis in chest wall tumours. World J Surg Oncol 2007; 5: 15.
8) Hayes AJ, Alexander N, Clark MA, Thomas JM Elastofibroma: a rare soft tissue tumour with a pathognomonic anatomical location and clinical symptom. Eur J Surg Oncol 2004; 30: 450-3.
9) Malghem J, Baudrez V, Lecouvet F, Lebon C, Maldague B, Vande Berg B. Imaging study findings in elastofibroma dorsi. Joint Bone Spine 2004; 71: 536-41.
10) Jarvi O, Saxen AE. Elastofibroma dorsi. Acta Pathol Microbiol Scand 1961; 51: 83-4.
11) Ochsner JE, Sewall SA, Brooks GN, Agni R. Best cases from the AFIP: Elastofibroma dorsi. Radiographics 2006; 26: 1873-6.
12) Tepe M, Polat MA, Çalışır C, İnan U, Bayav M. Prevalence of elastofibroma dorsi on CT: Is it really an uncommon entity? Acta Orthopaedica et Traumatologica Turcica 2019; 53; 195-8.
13) Kourda J, Ayadi-Kaddour A, Merai S, Hantous S, Miled KB, Mezni FE et al. Bilateral elastofibroma dorsi. A case report and review of the literature. Orthop Traumatol Surg Res 2009; 95: 383-7.
14) Prete PE, Henbest M, Michalski JP, Porter RW. Intraspinal elastofibroma. A case report. Spine 1983; 8: 800-2.
15) Petersson F. Elastofibroma in ligamentum flavum in a patient with symptoms of spinal stenosis: yet another case report. Int J Surg Pathol 2010; 18: 75-6.
16) Kapff PD, Hocken DB, Simpson RH. Elastofibroma of the hand. J Bone Joint Surg Br 1987; 69: 468-9.
17) Bae SJ, Shin MJ, Kim SM, Cho KJ. Intraarticular elastofibroma of the shoulder joint. Skeletal Radiol 2002; 31: 171-4.
18) Cross DL, Mills SE, Kulund DN. Elastofibroma arising in the foot. South Med J 1984; 77: 1194-6.
19) Battaglia M, Vanel D, Pollastri P, Balladelli MA, Alberghini M, Staals EL et al. Imaging patterns in elastofibroma dorsi. Eur J Radiol 2009; 72: 16-21.
20) Enjoji M, Sumiyoshi K, Sueyoshi K. Elastofibromatous lesion of the stomach in a patient with elastofibroma dorsi. Am J Surg Pathol 1985; 9: 233-7.
21) Hsu JK, Cavanagh HD, Green WR. An unusual case of elastofibroma oculi. Cornea 1997; 16: 112-9.
22) Brandser EA, Goree JC, El-Khoury GY. Elastofibroma dorsi: prevalence in an elderly patient population as revealed by CT. AJR Am J Roentgenol 1998; 171: 977-80.
23) Jarvi OH, Lamsimics PH. Subclinical elastofibroma in the scapular region in an autopsy series: additional notes on the aetiology and pathogenesis of elastofibroma pseudoneoplasm. Acta Pathol Microbiol Scand A 1975; 83: 87-10.
24) Gündoğdu E. Elastofibroma Dorsi: Prevalence and Imaging features on Multidetector Computed Comography. Ankara Eğt Arş Hast Derg 2017; 50: 26-31.
25) El Hammoumi M, Qtaibi A, Arsalane A, El Oueriachi F, Kabiri el H. Elastofibroma dorsi: clinicopathological analysis of 76 cases. Korean J Thorac Cardiovasc Surg 2014; 47: 111-6.
26) Chandrasekar CR, Grimer RJ, Carter SR, Tillman RM, Abudu A, Davies AM et al. Elastofibroma dorsi: an uncommon benign pseudotumour. Sarcoma 2008; 2008: 756565.
27) Briccoli A, Casadei R, Di Renzo M, Favale L, Bacchini P, Bertoni F. Elastofibroma dorsi. Surg Today 2000; 30:147-52.
28) Giannotti S, Bottai V, Dell’osso G, Bugelli G, Cazzella N, Guido G. Elastofibroma dorsi: case series of a rare benign tumour of the back. Eur J Orthop Surg Traumatol 2013; 23: 643-5.
29) Ulaner G, Hwang S, Landa J, Lefkowitz RA, Panicek DM. Musculoskeletal tumors and tumor-like conditions: common and avoidable pitfalls at imaging in patients with known or suspected cancer. International Orthopaedics 2013; 37:871-6.
30) Sarici IS, Basbay E, Mustu M, Eskut B, Kala F, Ağaçoğlu F et al. Bilateral elastofibroma dorsi: A case report. Int J Surg Case Rep 2014; 5: 1139-41.
21
Current Thoracic Surgery-Volume 8 Number 1 p: 16-22
31) Naylor MF, Nascimento AG, Sherrick AD, McLeod RA. Elastofibroma dorsi: radiologic findings in 12 patients. AJR Am J Roentgenol 1996; 167: 683-7.
32) Tamimi MI, Sesma SP, Perez LA, Martinez MJ, Vazquez ML, Tamimi F. Sensitivity and positive predictive value of magnetic resonance imaging in the diagnosis of elastofibroma dorsi: review of fourteen cases. J Shoulder Elbow Surg 2013; 22: 57-63.
33) Başaran C, Dönmez FY, Öztürk A, Tarhan NÇ. Elastofibroma Dorsi’de MRG Bulguları. Fırat Tıp Dergisi 2009; 14: 65-8.
34) Elitez B, Aşkın A, İsnaç F, Demirdal ÜS, Güvendi E. Bir Sırt Ağrısı Sebebi Olarak Elastofibroma Dorsi: Olgu Sunumu Şişli Etfal Hastanesi Tıp Bülteni 2017; 51:165-8.
35) Hoffman JK, Klein MH, McInerney VK. Bilateral elastofibroma: a case report and review of the literature. Clin Orthop Relat Res 1996; 325: 245-50.
36) Parratt MT, Donaldson JR, Flanagan AM, Saifuddin A, Pollock RC, Skinner JA et al. Elastofibroma dorsi: management, outcome and review of the literature. J Bone Joint Surg Br 2010; 92: 262-6.
37) Freixinet J, Rodríguez P, Hussein M, Sanromán B, Herrero J, Gil R. Elastofibroma of the thoracic wall. Interact Cardiovasc Thorac Surg 2008; 7: 626-8.
38) Oliva MS, Smimmo A, Vitiello R, Meschini C, Muratori F, Maccauro G et al. Elastofibroma dorsi: What’s new? Orthop Rev (Pavia) 2020; 12: 8708.
39) Fukuda Y, Miyake H, Masuda Y, Masugi Y. Histogenesis of unique elastinophilic fibers of elastofibroma: ultrastructural and immunohistochemical studies. Hum Pathol 1987; 18: 424-9.
40) Jarvi OH, Saxen AE, Hopsu-Have VK, Wartiovaara JJ, Vaissalo VT. Elastofibroma-a degenerative pseudotumor. Cancer 1969; 23: 42-63.
41) Schepel JAC, Seldenrijk CA, van Ramshorst B. Elastofibroma: a familial occurrence. Eur J Surg 1998; 164: 557-8.
42) Malghem J, Baudrez V, Lecouvet F, Lebon C, Maldague B, Berg BV. Imaging study findings in elastofibroma dorsi. Joint Bone Spine Imaging study findings in elastofibroma dorsi 2004; 71: 536-41.



