

2Department of Radiology, Recep Tayyip Erdoğan University, School of Medicine, Rize, Turkey DOI : 10.26663/cts.2023.008
Summary
Background: Tube thoracostomy is the most commonly performed procedure in thoracic surgery. Followup with daily chest X-ray is performed for both pneumothorax patients and postoperative patients, but residual pneumothorax can often be missed on portable imaging in supine position. This study aimed to evaluate the efficacy and safety of ultrasonography and chest X-ray by comparing these two methods in chest tube removal and thus determine whether ultrasonography is suitable for routine use in thoracic surgery clinics.Materials and Methods: This prospective study included a total of 28 patients who underwent tube thoracostomy in our center between December 2019 and December 2020 due to spontaneous pneumothorax (n = 16) or after wedge resection for different indications (n = 12). Chest X-ray and thoracic ultrasonography were performed before and after chest tube removal. The efficacy of thoracic ultrasonography compared to chest X-ray in the detection of residual pneumothorax was evaluated using specificity, sensitivity, positive predictive value (PPV), and negative predictive value (NPV).
Results: Twenty-four (85.7%) of the patients were male. The mean age was 40.25 ± 19.75 years (median, 39.5). Tube thoracotomy was performed on the right side in 18 patients (64.3%). Ultrasonography before tube removal had a PPV of 50%, NPV of 100%, sensitivity of 100%, and specificity of 96.3%. After tube removal, ultrasonography had a PPV of 100%, NPV of 96%, sensitivity of 75%, and specificity of 100%.
Conclusions: The results of our comparison of chest X-ray and thoracic ultrasonography examinations performed before and after chest tube removal showed that thoracic ultrasonography was as effective as chest X-ray and can be used safely in clinics.
Introduction
Tube thoracostomy (TT) is the most commonly performed procedure in thoracic surgery. It is among the most frequent interventions for preventing potentially fatal complications and postoperative pneumothorax (PTX) in particular. However, standard management guidelines for this procedure are still lacking. Different centers have varying practices regarding approaches to reduce morbidity, follow-up protocols, the timing of chest tube removal, and length of hospital stay [1,2]. Clinical follow-up of both PTX patients and postoperative patients is performed with daily chest X-ray (CXR), but many cases of PTX may be missed in portable imaging acquired in the supine position [3,4]. Although the most effective method of monitoring for PTX is computed tomography (CT), its use in daily practice is limited for several reasons, including that it is costly, involves intense radiation exposure, and is not portable, which causes difficulties in patient transport [4-7]. Thoracic ultrasonography (US) has been used in the evaluation of PTX for 30 years, and with recent advances in technology, it has become much more sensitive than CXR in the diagnosis of PTX [8,9]. Especially in recent years, its superiority over CXR in the detection of pathologies such as PTX and hemothorax in patients with chest trauma has been recognized. Thoracic US was also reported to be effective in demonstrating drain position during and after TT [10-12]. Advantages of bedside US include that it is easy to perform, can be readily repeated, is portable, has superior sensitivity and specificity, is low-cost, does not cause radiation exposure, and effectively guides the decision to remove a chest tube [1]. Despite the many articles on this subject in the literature, there has been little research on thoracostomy tube removal.This prospective study aimed to evaluate the efficacy and safety of US and CXR by comparing these two methods in chest tube removal and thereby determine whether US is suitable for routine use in thoracic surgery clinics.
Methods
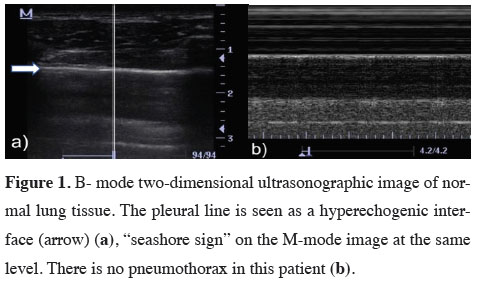
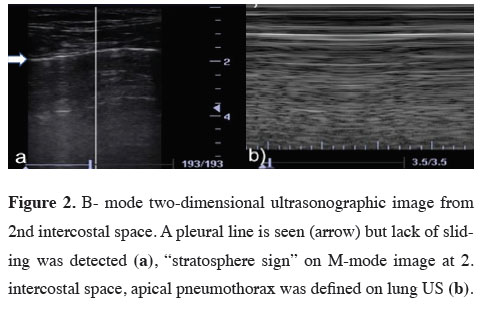
A total of 28 patients who underwent TT in the thoracic surgery department of the Recep Tayyip Erdoğan University Faculty of Medicine Training and Research Hospital between December 2019 and December 2020 were included in the study. Of these, 16 patients had spontaneous PTX and 12 underwent wedge resection for different indications. Patients under 18 years of age, pregnant women, patients who underwent TT due to trauma, and patients who underwent anatomical resection (lobectomy and segmentectomy) due to infectious or malignant causes were not included in the study.The decision to remove the chest tubes was made upon observing reduced oscillation in the drain and the resolution of the air leak. On the day of removal, the tube was clamped for 6 hours, during which CXR and concurrent bedside US were performed. Three hours after drain removal, CXR and concurrent US examination were repeated once more. All CXRs were obtained with the patient standing. Bedside US was performed at the second and third intercostal spaces on the midclavicular line with the patient in supine position with head raised 45 degrees. All US examinations were done by the same radiologist who was experienced in thoracic radiology and had not seen the patients’ CXRs. The procedure was performed in the longitudinal plane using a high-frequency superficial probe (DC-3; Mindray; 5-10 MHz). The appearance of lung lines and the presence of artifacts in the aerated lung tissue were evaluated in B-mode US imaging. The “lung sliding” sign was interpreted as lung expansion. Using the same device in the same area, the presence or absence of movement with the expansion of the lung tissue was examined under M-mode imaging. The “sandy beach” sign confirmed lung expansion, while the “stratosphere” sign was accepted as an indicator of air in the pleural space (Figure 1,2) [14].
 Click Here to Zoom |
Figure 1: B- mode two-dimensional ultrasonographic image of normal lung tissue. The pleural line is seen as a hyperechogenic interface (arrow) (a), “seashore sign” on the M-mode image at the same level. There is no pneumothorax in this patient (b). |
 Click Here to Zoom |
Figure 2: B- mode two-dimensional ultrasonographic image from 2nd intercostal space. A pleural line is seen (arrow) but lack of sliding was detected (a), “stratosphere sign” on M-mode image at 2. intercostal space, apical pneumothorax was defined on lung US (b). |
Approval for this prospective, single-center descriptive study was obtained from the Clinical Research Ethics Committee of Recep Tayyip Erdoğan University Faculty of Medicine Training and Research Hospital (date: September 3, 2020; decision no: 2020/193).
Statistical Analysis
Statistical analyses were performed using R Software version 4.0.3 (R foundation, Vienna, Austria). The data were presented as number and percentage values for categorical variables and as mean and standard deviation for numerical variables. The efficacy of US in the evaluation of lung expansion was assessed by calculating its sensitivity, specificity, negative predictive value (NPV), and positive predictive values (PPV) compared to CXR.
Results
Of the 28 patients who underwent TT in this study, 24 (85.7%) were men and 4 (14.3%) were women. The mean age was 40.25 ± 19.75 years (range, 19-75) and the median age was 39.5. TT was performed on the right side in 18 patients (64.3%) and on the left side in 10 patients (35.7%). The indication for TT was spontaneous PTX in 16 (57.1%) of the patients, while the other 12 patients (42.9%) underwent TT after surgery. Of these surgical procedures, 5 (41.6%) were video-assisted thoracoscopic surgery (VATS) wedge resection for lung biopsy, 4 (33.4%) were VATS bullectomy, and 3 (25%) were VATS metastasectomy. The mean length of hospital stay was 7.71 ± 2.92 days for nonsurgical patients (those who underwent TT due to pneumothorax) and 8.07 ± 2.87 days (median: 7) among postoperative patients (wedge resection) (Table 1).Table 1: Characteristics of patients (n = 28).
The chest tube was clamped for 6 hours before removal, during which CXR and US examinations were performed. In our comparison of the methods when used before thoracostomy tube removal, the US had a PPV of 50%, NPV of 100%, sensitivity of 100%, and specificity of 96.3% (Table 2). Of the 28 patients, PTX was detected in 1 patient with CXR, whereas 2 patients were evaluated as having PTX according to the US. This was due to the presence of apical bullae in one of the patients whose US findings were interpreted as PTX, which is the reason for the 50% PPV. In the comparison made after tube removal, the US had a PPV of 100%, NPV of 96%, sensitivity of 75%, and specificity of 100% (Table 3). PTX was detected on CXR in 4 patients after tube removal. PTX was detected in 3 of these patients in the US. Two of these 4 patients were followed with nasal oxygen for 24 hours and discharged, while the chest tubes were replaced in the other 2 patients.
Discussion
Thoracic US is an important post-trauma imaging modality, having 95% sensitivity and 100% specificity in the diagnosis of PTX and hemothorax in trauma patients [13,15]. Although there are many publications about the use of the US in the diagnosis of trauma patients, very few studies have investigated its effectiveness in planning thoracostomy tube removal [5,6,16]. In general, studies have only focused on the diagnostic efficacy of the US and how it compares to CXR.Thoracic US is helpful in many stages of the TT procedure. Authors have discussed the efficacy of using the US at every stage, from preventing intercostal injury and tube malposition during placement to daily monitoring and deciding the most appropriate time for tube removal [11,12,17]. Lavingia et al emphasized that physicians can easily make these evaluations with basic US training [18].
Complications that may occur after tube removal can be listed simply as PTX recurrence and the development of hemothorax. Hernandez et al. reported a three-fold increase in cost when these complications occur after tube removal. They emphasized that increased cost was a result of missing the findings on CXR, whereas this rate was quite low in patients evaluated with US [19]. However, in our series, there were no patients with recurrent PTX or fluid collection that was missed on CXR but detected by the US.
US evaluation of lung expansion is based on the movement of the pleural line. In the presence of pneumothorax, movement of the pleura is not observed in B-mode imaging. In M mode, parallel lines form in the absence of movement, which is called a barcode or stratosphere sign. As a control, when the lower intercostal spaces or symmetrical lung space are evaluated in M mode, the parallel line structure is disrupted and a complex dotted appearance occurs upon lung expansion (sandy beach sign) [20].
Studies have suggested that the US of the chest has similar accuracy to plain radiography. The main advantages of US are that it can be performed at the bedside, has a short evaluation time, and does not involve ionizing radiation [14]. In addition to emergency medical units, the use of the US in thoracic surgery wards to examine patients during PTX follow-up and before thoracostomy tube removal has been discussed in the literature in recent years [21]. In a 2018 study conducted with 50 patients, it was suggested that follow-up imaging of patients after tube removal could be performed by the US [20]. Galbosis et al reported in their study that residual air that was undetectable on plain radiography could be demonstrated by lung US. Only patients with residual PTX detected by the US required tube replacement [22]. In recent years, we have started routinely using thoracic US in the diagnosis and treatment of effusions that require intervention in patients with hemothorax, as well as for other thoracic catheterizations that require prior marking. With support from experienced radiologists, we have also started increasing our experience with the use of bedside US in chest tube removal to protect our patients from additional radiation.
Regarding the use of US during chest tube removal, a meta-analysis of thoracic US emphasized that the adequacy of US depends on the examiner [9]. In our study, we also attempted to identify factors that may affect the results of evaluations made by the US. The presence of the chest tube makes it difficult to assess pleural movement and reduces its prominence in the US. Before the procedure, the patient’s head should be elevated and time allowed for any air present in the pleural space to move cranially. Turning on the device after adjusting the patient’s position provides the optimal time required before starting the procedure. Observing the pleural movement of the fellow lung can prevent false positive results. US evaluation of pleural movement is difficult in patients with bullae, diffuse interstitial lung diseases, or a prior history of chest tube placement. Movements of the chest wall and the effect of heart pulsation on the left lung can cause an appearance that mimics the pleural movement and lead to false negative results [8,16,20,22].
The main limitation of our study is that the despite this being a prospective study, we were unable to conduct multivariate analyses because of the small number of patients. Due to the COVID-19 pandemic, we limited the sample size to avoid unnecessary risks to the health of both patients and physicians.
In conclusion, the data obtained in our study support the conclusion that US is as effective as CXR in thoracostomy tube removal and the evaluation of residual pneumothorax. Larger prospective studies with homogeneous groups are needed in this regard. The most important advantages of bedside US are that it is easy to perform, involves no radioactive adverse effects, reduces the need for patient transport, and can provide a rapid diagnosis. Apart from residual PTX, its ability to reveal fluid collections, especially in postoperative patients, is also valuable.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Ethics approval
Approval for this prospective, single-center descriptive study was obtained from the Clinical Research Ethics Committee of Recep Tayyip Erdoğan University Faculty of Medicine Training and Research Hospital (date: September 3, 2020; decision no: 2020/193).
Authors’ contributions
KT, NH; conceptualized and designed the study, collected analyzed and interpreted the patient data, GS, HT; collected and analyzed data and revised the final version of the manuscript. All authors read and approved the final manuscript.
Reference
1) Satoh Y. Management of chest drainage tubes after lung surgery. Gen Thorac Cardiovasc Surg 2016; 64: 305–8.
2) de Abreu EM, Machado CJ, Pastore Neto M, de Rezende Neto JB, Sanches MD. The impact of a chest tube management protocol on the outcome of trauma patients with tube thoracostomy. Rev Col Bras Cir 2015; 42: 231-7.
3) Martino K, Merrit S, Boyakye K, Sernas T, Koller C, Hauser CJ et al. Prospective randomized trial of thoracostomy removal algorithms. J Trauma 1999; 46: 369-71.
4) Rowan KR, Kirkpatrick AW, Liu D, Forkheim KE, Mayo JR, Nicolaou S. Traumatic pneumothorax detection with thoracic US: correlation with chest radiography and CT initial experience. Radiology 2002; 225: 210.
5) Kwan RO, Mirafor E, Yeung L, Strumwasser A, Victorino GP. Bedside thoracic ultrasonography of the fourth intercostal space reliably determines safe removal of tube thoracostomy after traumatic injury. J Trauma Acute Care Surg 2012; 73: 1570-5.
6) Tanoue LT. Computed tomography—an increasing source of radiation exposure. Pulm Dis 2012; 2009: 154-5.
7) Ball CG, Kirkpatrick AW, Laupland KB, Fox DL, Litvinchuk S, Dyer DM et al. Factors related to the failure of radiographic recognition of occult posttraumatic pneumothoraces. Am J Surg 2005; 189: 541.
8) Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17: 11-17.
9) Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest 2011; 140: 859Y866.
10) Martin M, Schall CT, Anderson C, Kopari N, Davis AT, Stevens P et al. Results of a clinical practice algorithm for the management of thoracostomy tubes placed for traumatic mechanism. Springerplus 2013; 2: 642.
11) Menegozzo CAM, Utiyama EM. Steering the Wheel towards the standard of care: proposal of a step-by-step ultrasound-guided emergency chest tube drainage and literature review. Int J Surg 2018; 56: 315-9.
12) Jenkins JA, Gharahbaghian L, Doniger SJ, Bradley S, Crandall S, Spain DA et al. Sonographic Identification of Tube Thoracostomy Study (SITTS): Confirmation of Intrathoracic Placement. West J Emerg Med 2012; 13: 305-11.
13) Staub LJ, Biscaro RRM, Kaszubowski E, Maurici R. Chest ultrasonography for the emergency diagnosis of traumatic pneumothorax and hemothorax: a systematic review and meta-analysis. Injury 2018; 49: 457-66.
14) Zanobetti M, Poggioni C, Pini R. Can chest ultrasonography replace standard chest radiography for evaluation of acute dyspnea in the ED? Chest 2011; 139: 1140-7.
15) Ianniello S, DiGiacomo V, Sessa B, Miele V. First-line sonographic diagnosis of pneumothorax in major trauma: accuracy of e-FAST and comparison with multidetector computed tomography. Radiol Med 2014; 119: 674-80.
16) Karagöz A, Ünlüer EE, Akçay O, Kadioǧlu E. Effectiveness of bedside lung ultrasound for clinical follow-up of primary spontaneous pneumothorax patients treated with tube thoracostomy. Ultrasound Q 2018; 34: 226-32.
17) Salamonsen M, Dobeli K, McGrath D, Readdy C, Ware R, Steinke K et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology 2013; 18: 942-7.
18) Lavingia KS, Soult MC, Collins JN, Novosel TJ, Weireter LJ, Britt LD. Basic ultrasound training can replace chest radiography for safe tube thoracostomy removal. Am Surg 2014; 80: 783-6.
19) Hernandez MC, Laan DV, Zimmerman SL, Naik ND, Schiller HJ, Aho JM. Tube thoracostomy: increased angle of insertion is associated with complications. J Trauma Acute Care Surg 2016; 81: 366-70.
20) Patella M, Saporito A, Puligheddu C, Mongelli F, Regina D La, Pini R et al. Lung ultrasound to detect residual pneumothorax after chest drain removal in lung resections. Ann Thorac Surg 2018; 105: 1537-42.



