

2Department of Thoracic Surgery, Sincan Training and Research Hospital, Ankara, Turkey DOI : 10.26663/cts.2023.0023
Summary
Background: Chest wall tumors can be malignant and benign and present as primary or metastatic lesions. For the definitive treatment of malignant thoracic wall tumors, the surgical margin should be established at a distance of at least 4 cm from the tumor. A 1-2 cm distance from the tumor is often sufficient in benign or low-grade malignancies. Repairing the deformity with prosthetic materials in 3 or more rib resections (>30 cm) is recommended. In resections containing four or more ribs, the mesh should be supported with metallic rib bars screwed to the periphery of the defect.Materials and Methods: Thoracic wall resection was performed on 285 patients between 2008 and 2019 in the Department of Thoracic Surgery of Ankara Atatürk Sanatoryum Training and Research Hospital. Repair with prosthetic graft was performed in 70 cases, and thoracic wall resection was performed in 215 patients without using mesh. The results of 50 patients who underwent thoracic wall reconstruction with a prosthetic graft were evaluated retrospectively.
Results: The female/male ratio in those using mesh is 0.47; the mean age is 52.5 (14-76 years); the tumor size (mean long diameter) is 11 cm (4-18 cm); the number of removed ribs is 2.5 (1-5 pieces) is Sternal resection was performed in 2 patients, sternum resection in 1 patient, left clavicle partial resection, right clavicle partial resection, and first and second ribs of the left side resection. Partial excision of the clavicle and first rib was performed in 1 patient. Polypropylene mesh in 28, PTFE mesh in 20, and polyglactin mesh in 2 patients were used. The mean postoperative hospital stay was 10.6 days (2-58 days), and the mean follow-up period was 16.6 months (0-96 months, median 7 months). Complications developed in 10 patients (20.0%). Three patients underwent revision surgery; one was operated on for empyema at four months, and the patch was removed. The others were performed at the 16th and 30th months due to recurrence. Mortality developed in 4 patients in long-term follow-ups.
Conclusions: Polypropylene mesh can cause wrinkles and folds as it shows less stretch when suturing than Polytetrafluoroethylene (PTFE). In addition to the difficulties of providing a smooth surface, it also causes the passage of fluid and air in the pleural space from the pores to the subcutaneous space. PTFE patches are frequently used, non-permeable, flexible, high tissue compatibility, durable and robust, but poor body wall integration has been reported. Polypropylene and PTFE mesh comparison results are similar to the literature. Suppose a significant defect (>30 cm2) exists in patients who have undergone thoracic wall resection; reconstruction should be performed to stabilize the thoracic wall, prevent lung hernia, paradoxical breathing, mediastinal structures, and intrathoracic dislocation of the scapula, and provide aesthetically appropriate rib cage contours.
Introduction
Chest wall entirety and stability are the primary aspects that protect intrathoracic organs and adequate respiratory function. Following the resection of the rib or sternum with soft tissue, chest wall reconstruction commonly requires adding components to provide chest wall stability and coverage with well-vascularized soft tissue. Repairing skeletal stability is essential to diminish the negative effect on respiratory function [1,2]. The goals are avoidance of lung hernia, paradoxical chest wall motion, scapular impaction into the defect in posterior chest wall resections, and protection of the underlying mediastinal organs in anterior chest wall resections with providing an aesthetically pleasing chest contour. The location, size, depth of the defect, the viability of the surrounding tissue, and prior operative procedures determine the optimal approach to reconstruction [3]. Defects >5 cm in diameter or including >3 ribs should be reconstructed due to the increased risk of lung herniation and respiratory compromise from the paradoxical movement of the chest wall, particularly valid for anterolateral defects and total thickness resections[3,4]. The reconstruction of chest wall defects with prosthetic material depends upon defect size and location. The resections that include three or more ribs (more than 30 cm2) typically require reconstruction. Some apical-posterior defects, even 10 cm in size, do not require reconstruction because of the scapula and shoulder girdle support, except for defects lower than the fourth rib posteriorly, with the tip of the scapula at risk of entrapment[3,4]. The fundamental need for skeletal reconstruction for more minor defects has been inquired. [6,7].Synthetic mesh sutured beneath tension supplies an adequately rigid chest wall repair that decreases ventilator support and hospitalization [8]. Since using synthetic mesh, rigid reconstruction utilizing autologous rib grafts or semirigid fascial grafts has evolved less commonly; these require a donor site harvest. The superior synthetic mesh should be rigid enough to underestimate paradoxical chest wall motion, porous enough to allow tissue ingrowth, and malleable, radiolucent, and inert [9]. Autologous grafts may still be utilized if a synthetic mesh is not readily obtainable or the wound is at an increased risk of infection.
This study aims to evaluate surgical outcomes of chest wall reconstruction, focusing on synthetic materials in chest wall tumors regarding early mortality, complications, and length of hospital stay.
Methods
Between 2008 and 2019, 285 patients who underwent thoracic wall resection in our clinic were evaluated retrospectively. The patients were evaluated in age, gender, clinical features, the surgical method applied, defect size, prosthetic material, pathological diagnoses, length of hospital stay, complications, recurrence, and mortality. Patients whose adequate information could not be accessed in the system were excluded from the study.All patients were evaluated with preoperative chest x-ray and thorax CT. Thoracic CT-guided transthoracic biopsy was taken for patients without a previous pathological diagnosis. Pet CT in malignant patients was used for staging, and invasion was evaluated preoperatively with thoracic MR in patients with suspected close tissue invasion. CT and MR imaging determined the tumor's location, depth, and invading tissues. The margin for tumor resection was determined preoperatively, and which soft tissues, bone structures, and lung tissues should be removed. Bone and soft tissue were resected 3 cm away from the tumor, with the negative margin being the most critical. Because giant tumors often invade the thorax, ribs, pleura, and even some lung tissue, these components often need to be resected to achieve R0 incised margins.All patients were transferred to the ICU after the operation.
We operated on patients whose tumor was considered completely resectable as long as their general medical condition allowed surgery. All cases were reviewed by a multidisciplinary team (MDT) before surgery. Neo-adjuvant chemotherapy was prescribed according to the MDT decision, and MDT reassessed adjuvant treatments (chemotherapy and radiation) according to the primary diagnosis, surgical outcomes, and final pathological report. This study was approved by the Ankara Atatürk Sanatoryum Training and Research Hospital institutional Ethics Committee (No: 2710/2023).
Results
Chest wall resection was performed on 285 patients between January 2008 and December 2019 in the Department of Thoracic Surgery of Ankara Atatürk Sanatoryum Training and Research Hospital. Prosthetic grafts were used in 70 cases, and 215 patients underwent thoracic wall resection without using mesh. The results of fifty patients who underwent thoracic wall reconstruction with a prosthetic graft were included for evaluation retrospectively. Twenty patients who underwent prosthetic material were excluded from the study because they did not meet the inclusion criteria for reasons such as being unable to access their information or being unfollowed. Twenty patients were excluded due to did not meet the inclusion criteria.All patients were considered with chest X-rays and thorax CT. Thoracic CT-guided transthoracic biopsy was taken for patients without a diagnosis. PET/CT in malignant patients was used for staging, and invasion was estimated preoperatively with thoracic MR in patients with suspected, immediate tissue invasion. All patients received preoperative standard antibiotic prophylaxis.
The female/male ratio in those using mesh is 0.52; the mean age is 54.34 (14-76 years), and the tumor size is 10.66 cm (4-18 cm).
The most frequent malignant tumors were squamous cell carcinoma (n: 14, 28%), adenocarcinoma (n: 6, 12%), and chondrosarcoma (n: 4, 8%). Preoperative chemotherapy was administered in 19 malignant patients, and 27 had adjuvant chemotherapy.
The benign diseases were fibrous dysplasia (n: 5, 10%), osteochondroma (n: 1, 2%), tuberculosis (n: 1, 2%), (the diagnosis of the patients indicated in Table 1).
Table 1: Diagnosis of the patients who underwent chest wall reconstructions with synthetic mashes.
The mean long diameter and the number of removed ribs is 2.70 (1-5 pieces).In addition to chest wall resection, lobectomy was performed in 22 patients, thymectomy in 1 patient, and pericardial resection with the diaphragm in 1 patient. The sternum was resected in 4 patients, and the clavicle was resected in 2. Polypropylene mesh in 28 (56%), PTFE mesh in 20 (40%), and polyglactin mesh in 2 patients (4%) were used. The mean postoperative hospital stay was 9.87 days (2-39 days), and the mean follow-up period was 16.6 months (0-96 months, median 7 months).
Postoperative complications developed in 10 patients (20%). Wound infection developed with prolonged air leakage in 2 patients with PTFE mesh and was treated with debridement antibiotics and wound care. Chylothorax (n: 1) occurred on the 2nd day and was treated with conservative treatments. Serous wound drainage with subcutaneous emphysema (n: 2, polyprolene mesh) developed and was treated with conservative treatment. In contrast, empyema occurred in a patient with PTFE on the 9th day, and another had empyema in the fourth month. The first patient was treated with antibiotics and tube thoracostomy; the other patient occurred in the fourth month, underwent surgery for mesh removal. One patient with prolonged air leakage underwent bulla resection with VATS on the fifth day. One patient with polypropylene mesh due to bleeding (on the second day) underwent a re-thoracotomy to evacuate the hematoma.
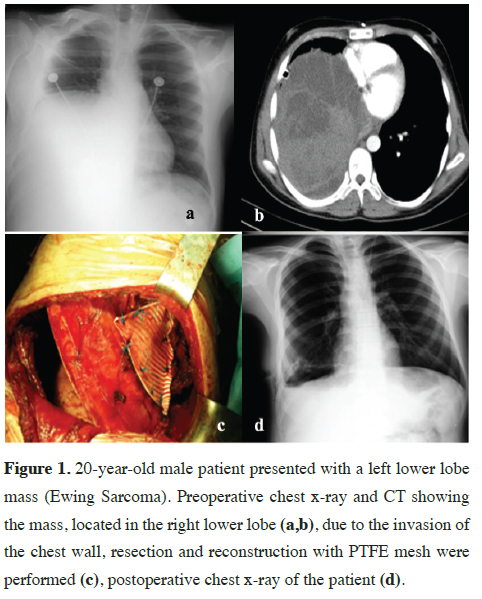
After falling from high, a 20-year-old male patient was referred to our hospital for hemopneumothorax, and a parenchymal mass with chest wall invasion was detected in exploration with thoracotomy incidentally. Chest wall resection and reconstruction were performed with PTFE mesh (Figure 1). The histopathological diagnosis was Ewing Sarcoma/ primitive neuroectodermal tumor.
Late revision surgery was performed for three patients; one was operated on due to empyema in the 4th month and mesh removed, and others were operated on for recurrence in the 16th and 30th months.
 Click Here to Zoom |
Figure 1: 20-year-old male patient presented with a left lower lobe mass (Ewing Sarcoma). Preoperative chest x-ray and CT showing the mass, located in the right lower lobe (a,b), due to the invasion of the chest wall, resection and reconstruction with PTFE mesh were performed (c), postoperative chest x-ray of the patient (d). |
A patient with squamous cell carcinoma had respiratory distress due to pulmonary thromboembolism on the 12th day and was deceased. In addition, three patients were deceased during follow-up. One died due to MHRS pneumonia after chemotherapy in the third month, one died due to respiratory distress due to recurrent thymoma in the sixth month, and another died in the ninth month due to lung adenocarcinoma metastasis to the contralateral lung and brain. Demographics and overall survival of the patients are mentioned in Table 2.
Discussion
A fundamental principle preliminary to the initiation of the chest wall reconstruction is a proper and thorough chest wall resection that leaves healthy, viable margins to which materials and tissues used in a reconstruction may be anchored securely. Detailed preoperative assessment for the spread of disease in patients with primary or metastatic malignancies is necessary before chest wall resection or reconstruction, especially for patients with breast and lung cancer locally invading the chest wall and in patients with metastatic disease to the ribs or sternum.The treatment principle of chest wall malignant tumors is to reach a negative margin by radical resection, extend the survival time, and decrease the mortality and postoperative recurrence rate [9,10]. Most patients undergoing primary chest wall resection will have some postoperative respiratory dysfunction. However, the severity is often less than that following trauma. Therefore, in extra to routine laboratory tests, pulmonary function tests (i.e., spirometry) should be acquired preoperatively to determine the patient's candidacy for the offered resection and to provide a comparison for postoperative comparison [11,12]. Negative margins are an important predictive factor for local recurrence rate. For high-grade, aggressive, or highly infiltrating malignancies, wide local excision with 4 cm margins is usually indicated; however, resection in an individual patient depends on the type of tumor and anatomic location. 1 to 2 cm margins are generally sufficient for benign processes and low-grade malignancies [2]. Repair of skeletal stability is critical to reducing the adverse effects on respiratory function. The purposes include preventing pulmonary herniation, paradoxical chest wall motion, scapular impaction into the defect during posterior chest wall resections and defense of underlying mediastinal organs in anterior chest wall resections by reaching an aesthetically pleasing chest silhouette [1]. Synthetic mesh sutured under tension supplies an adequately rigid chest wall repair that reduces ventilator reliance and hospital stay. While the recurrence rate in negative surgical margins is 10%, it increases to 75% in positive surgical margins. For this reason, resection margins should not be compromised with the concern of closing the defect [13]. In our study, recurrence developed in 4 (8%) patients in the thoracic wall resection and reconstructions performed by preserving the negative surgical margin. Two patients (4%) were re-operated for recurrence. Thymoma recurred in 1 patient (2%) and adenocarcinoma in the other patient; both were deceased.
Primary sarcoma and recurrent breast cancer mainly involve the chest wall. Primary chest wall tumors are frequently chondrosarcoma and fibrosarcoma, accounting for about 77.8% of all cases [14]. In the study, chest wall invasion and metastasis rates due to lung cancer were high in our patients who underwent chest wall reconstruction (42%). Chest wall reconstruction was performed in 1 patient (2%) due to breast cancer infiltration. We attribute that a small number of patients were operated on for breast cancer because our center is not multidisciplinary.
The preferred semirigid material for extensive chest wall repairs is synthetic mesh (e.g., polypropylene, polytetrafluoroethylene). Mesh is less rigid than polymethylmethacrylate (PMMA), which is rarely used [15-18] and economical but can extend in the long term with laxity developing at the repair site. Besides, when a mesh is positioned directly in contact with the viscera, such as the lung or exposed bowel (e.g. when the diaphragm requires partial resection), or when the operative site has been irradiated, the risk of complications is increased [20] Synthetic mesh is contraindicated in contaminated wounds.
In our current experience, we do not use methylmethacrylate grafts because it is allergic and more likely to be rejected by the body. In cases where we had thoracic wall resection, we preferred a PTFE patch as much as possible due to lower air and fluid permeability. Prolene mesh is permeable due to punched structure, so the area must be supported with a pedicled muscle graft.
Compared to Polytetrafluoroethylene, Polypropylene mesh causes wrinkles and folds due to less stretching while suturing and the difficulties of providing a smooth surface and the passage of fluid and air in the pleural space from the pores to the subcutaneous area (Figure 1). PTFE patches are frequently used, impermeable, flexible, high tissue compatibility, durable and robust, but poor body wall integration has been reported [21]. The study used polypropylene mesh in 56% of the patients, PTFE in 40% and polyglactin mesh in 4%.
The chest wall reconstruction must be tightly sutured to the surrounding bony chest wall using heavy non-absorbable sutures. We prefer to insert the mesh inlay to prevent lung herniation. In substantial defects involving four or more ribs, the mesh can be reinforced with a metallic rib strut that is screwed to the periphery of the defect. One or two struts are sufficient to increase the rigidity of the chest wall. The mesh is then suspended to the metallic strut with heavy sutures. Once the chest wall defect is stabilized, wellvascularized soft tissue coverage should be provided to minimize the risk of graft exposure and potential infection.
Different prosthetic materials have been defined for reconstruction to provide good pulmonary function, protect the intrathoracic organs from infection and trauma, and maintain cosmetic integrity. However, they might be rejected, displaced or cause septic complications such as foreign body reaction [19].Although the frequency of wound complications varies between 10-20% in the first 90 days, journals report that 5% of patients require the removal of the prosthesis [22]. In the study, complications developed in 10 patients (20%) and the mesh was removed in 2 patients (4%). Our results were found to be compatible with the literature.
They are generally metallic materials designed to bridge costal and sternal defects. It provides more physiological costal respiratory movements than methyl methacrylate and other patch prostheses. They often must be combined with other mucocutaneous flaps or meshes to wrap the chest wall and isolate the pleural space. As a disadvantage, fracture or displacement can be seen in titanium implants with a frequency of up to 44% [23].
In conclusion, reconstructing extensive chest wall defects with or without thoracic wall resection could be challenging. The reconstruction should be performed by adhering to biomimetic principles, in which anatomy is esteemed, the function is preserved, optimal reconstructive materials are chosen, and a multidisciplinary approach to complex reconstruction is undertaken. After an R0 chest wall resection, first skeletal stability must be established with prosthetic or bioprosthetic meshes or a combination of both. Soft tissue coverage must be achieved using one of multiple available rotational, advancement or free flaps. With the new immediate changes in biodegradable scaffoldings and innovation in surgical techniques, outcomes for extensive chest wall reconstruction are expected to continue to improve.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect
to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research
and/or authorship of this article.
Ethics approval
This study was approved by the Ankara Atatürk Sanatoryum
Training and Research Hospital institutional Ethics
Committee (Number 2710: Approved on May 2023).
Authors’ contribution
SH: conceptualized and drafted the article, wrote the
paper; NS,LNÜA: drafted the article, collected and analyzed
data; PB,GF,SŞEG: revised the final version of
the manuscript. All authors approved the final manuscript
as submitted and agree to be accountable for all
aspects of the work.
Reference
1) Bakri K, Mardini S, Evans KK, Carlsen BT, Arnold PG. Workhorse
flaps in chest wall reconstruction: the pectoralis major,
latissimus dorsi, and rectus abdominis flaps. Semin Plast Surg
2011; 25:43-54.
2) Clemens MW. Surgical management of chest wall tumors. In:
UpToDate, Collins KA (Ed), UpToDate, Waltham, MA. (Accessed
on July 21, 2021.)
3) Sanna S, Brandolini J, Pardolesi A, Argnani D, Mengozzi M,
Dell'Amore A et al. Materials and techniques in chest wall reconstruction:
a review. J Vis Surg 2017; 3: 95-109.
4) Seder CW, Rocco G. Chest wall reconstruction after extended
resection. J Thorac Dis 2016; 11: 863-71.
5) Yağcı T, Üçvet A, Çimen Güvenç E, Yoldaş B, Gürsoy S. Management
of chest wall tumors: a single-center experience. Curr
Thorac Surg 2017; 2: 46-9.
6) Butterworth JA, Garvey PB, Baumann DP, Zhang H, Rice DC,
Butler CE. Optimizing reconstruction of oncologic sternectomy
defects based on surgical outcomes. J Am Coll Surg 2013;
217: 306-16.
7) Hanna WC, Ferri LE, McKendy KM, Turcotte R, Sirois C,
Mulder DS. Reconstruction after major chest wall resection:
can rigid fixation be avoided? Surgery 2011; 150: 590-7.
8) Kroll SS, Walsh G, Ryan B, King RC. Risks and benefits of using
Marlex mesh in chest wall reconstruction. Ann Plast Surg
1993; 31: 303-6.
9) le Roux BT, Shama DM. Resection of tumors of the chest wall.
Curr Probl Surg 1983; 20: 345-86.
10) Dai Z, Maihemuti M, Sun Y, Jiang R. Resection and reconstruction
of huge tumors in the chest wall. J Cardiothorac Surg
2022; 17: 116-23.
11) Azarow KS, Molloy M, Seyfer AE, Graeber GM. Preoperative
evaluation and general preparation for chest-wall operations.
Surg Clin North Am 1989; 695: 899-910.
12) Mansour KA, Thourani VH, Losken A, Reeves JG, Miller JI, Jr
Carlson GW et al. Chest wall resections and reconstruction: a
25-year experience. Ann Thorac Surg 2002; 73: 1720-6.
13) Fong YC, Pairolero PC, Sim FH, Cha SS, Blanchard CL, Scully
SP. Chondrosarcoma of the chest wall: a retrospective clinical
analysis. Clin Orthop Relat Res 2004; 427: 184-9.
14) Warzelhan J, Stoelben E, Imdahl A, Hasse, J. Results in surgery
for primary and metastatic chest wall tumors. Eur J Cardiothorac
Surg 2001; 19: 584-8.
15) Arnold PG, Pairolero PC. Chest-wall reconstruction: an account
of 500 consecutive patients. Plast Reconstr Surg 1996;
98: 804-10.
16) Rathinam S, Venkateswaran R, Rajesh PB, Collins FJ. Reconstruction
of the chest wall and the diaphragm using the inverted
Y Marlex methylmethacrylate sandwich flap. Eur J Cardiothorac
Surg 2004; 26: 197-201.
17) Lardinois D, Müller M, Furrer M, Banic A, Gugger M, Krueger
T et al. Functional assessment of chest wall integrity after
methylmethacrylate reconstruction. Ann Thorac Surg 2000;
69: 919-23.
18) McKenna RJ Jr, Mountain CF, McMurtrey MJ, MJ, Larson D,
Stiles QR. Current techniques for chest wall reconstruction: expanded
possibilities for treatment. Ann Thorac Surg 1988; 46: 508.
19) Aksoy Y, Çıtak N, İşgörücü Ö, Obuz Ç, Sayar A. The treatment
of foreign body reaction and inflammation caused by prosthetic
material with surgery and negative pressure wound therapy.
Curr Thorac Surg 2019; 4: 140-2.
20) Butler CE, Langstein HN, Kronowitz SJ. Pelvic, abdominal,
and chest wall reconstruction with AlloDerm in patients at
increased risk for mesh-related complications. Plast Reconstr
Surg 2005; 116: 1263-75.
21) Deschamps C, Tirnaksiz BM, Darbandi R, Trastek, VF, Allen MS,
Miller DL et al. Early and long-term results of prosthetic chest
wall reconstruction. J Thorac Cardiovasc Surg 1999; 117: 588-92.



