

2Department of Thoracic and Cardiovascular Surgery, Edgardo Rebagliati Martins National Hospital, Lima, Peru
3Postgraduate Area, School of Medicine, Universidad San Martín de Porres, Lima, Peru DOI : 10.26663/cts.2023.0024
Summary
Background: Bronchopleural fistula (BPF) is a complication with a great impact on morbidity, mortality and survival in postoperative pneumonectomy patients. Our study aimed to determine the associated clinical-surgical characteristics of the BPF in a Peruvian cohort of patients with BPF following pneumonectomy.Materials and Methods: Cross-sectional, and retrospective study during January 2015-June 2020 in a Peruvian National Hospital. Medical records and operative reports were reviewed, and all patients over age (>18 years) and with all-cause pneumonectomy were considered. The variables were grouped into pneumonectomies with and without BPF, and the characteristics were grouped as a baseline, clinicalsurgical and postoperative.
Results: Among fifty-seven patients who underwent pneumonectomy, 28% presented BPF. The mean age was 38.3 ± 15 years, and 62.5% were male. The main pathologies associated with BPF were posttuberculosis fibrocavitary sequelae (62.5%), pulmonary hydatidosis (18.8%), and post-surgical cavitary sequelae (12.5%). 81.3% were left BPF (p = 0.342) and 100% were related to postoperative empyema (p < 0.05). The mortality associated with BPF was 6.25%, and in the non-BPF group it was 4.87% (p = 0.098). Characteristics that were statistically associated with the presence of bronchopleural fistula were low predicted forced expiratory volume (FEV1) (56.3% vs. 31.7%; p = 0.046), preoperative empyema (37.5% vs. 2.4%; p = 0.001), postoperative empyema (100% vs. 9.8%; p = 0.000) and operative time (450.69 min vs. 367.73 min; p = 0.032).
Conclusions: There are a series of factors associated with the presence of patients with BPF following pneumonectomy, many of them mainly related to operative and postoperative events, which is an important predictor of associated complications in our Peruvian population.
Introduction
Bronchopleural fistula (BPF) is a complication with a great impact on morbidity and mortality (6-71%) in postoperative pneumonectomy patients [1-3], and is defined as the communication of a bronchus into the pleural cavity. Many cases are more frequently associated with patients with BPF following pneumonectomy than with other pulmonary resections [4]. It has been reported in 20% of postoperative patients with complete lung resection due to benign etiology (Mycobacterium tuberculosis) and 4.5% in lung cancer [5,6]. The diagnosis of post- pneumonectomy BPF can be early or late and leads to a chronic situation that weakens the patient, leading to malnutrition problems, risk of reactivating tuberculous infections with Multidrug- Resistant germs (MDR), and even leading to death in 10% [1,4,7]. Among the main factors widely described are a history of pulmonary tuberculosis (35%), malnutrition (21%), and right pneumonectomy (15%) [1-3,8-11].Latin America has with the highest rate of indication of extensive tuberculous pulmonary resections in our environment. This has led to the need to implement operating rooms strictly for this type of surgery such as the one in our institution, with higher rates of BPF than in lung cancer surgeries. Although the surgical technique has progressed, which has generated a decrease in the incidence of BPF, it continues to generate great concern for the thoracic surgeon due to the multifactorial origin of this complication. Therefore, our study aimed to determine the associated clinical-surgical characteristics of the BPF in a Peruvian cohort of patients who underwent pneumonectomy.
Methods
Design, population, and sample sizeA cross-sectional and retrospective study was conducted. Fifty-seven post-pneumonectomy patients previously evaluated by the thoracic surgery service of a Hospital Nacional from Peru during January 2015-June 2020 were studied. Inclusion criteria were the majority of age (>18 years), and pneumonectomy due to different etiologies (tuberculosis, pulmonary hydatidosis, malignant pulmonary tumor, benign pulmonary tumor, severe pulmonary sequelae, and others) and that ended with or without BPF. All patients who underwent pneumonectomy with or without BPF outside the study period and without complete information were excluded.
Data collection and study variables
Data collection was retrospective, and medical records
and operative reports were evaluated using a data collection
form. Subsequently, the data were tabulated in
an orderly manner and respected the chronological order
of the patients. A checklist was used, and to avoid
any tabulation bias, each of the data obtained was double-
checked. The study variables were divided into 2
groups (Pneumonectomies with BPF vs. Pneumonectomies
without BPF) and the characteristics evaluated
were baseline (age, sex, comorbidities, smoking and
alcoholism history), clinical-surgical (pulmonary tuberculosis,
type of surgical indication, malnutrition, anemia,
hypoalbuminemia, chronic obstructive pulmonary
disease (COPD), diabetes mellitus, forced expiratory
volume during the first second (FEV1 predicted), overall
health (ASA >II), mechanical ventilation greater than
2 hours, pleural empyema pre and post-surgery, pleural
empyema pre and post-surgery (pleural empyema pre
and post-surgery), mechanical ventilation greater than
24 hours, pre-and postoperative pleural empyema,
neoadjuvant therapy and postoperative pulmonary infection,
location and type of pneumonectomy, type of
suture, operative bronchial closure technique (SWEET,
separate loose stitch plication of the cartilaginous bronchial
portion over the membranous part, and MABIT,
bloc closure of the bronchial stump with arterial and/or
venous vessels from the pulmonary hilum), bronchial
stump coverage, lymph node dissection, residual tumor
in bronchial stump, operative time, intraoperative bleeding
and the presence of BPF). The present investigation
respected ethical principles, and patient anonymity, and
since it was a retrospective study, it was not necessary
to implement informed consent. The study protocol
was approved by the ethics committee of the Universidad
de San Martín de Porres (USMP-00456/2022)
and the Hospital Nacional Hipólito Unánue (NIT2309-
06/2022), Lima, Peru.
b>Statistical Analysis
Statistical calculations were based on descriptive analyses
with continuous variables (Mean and Standard Deviation)
and categorical variables (Counts and percentages).
Statistical tests were based on Chi-square, Student's
t-test, Fisher's exact test, and the Mann-Whitney U test.
The entire statistical analysis of the data was performed
with the statistical programs Microsoft Excel 2020 and
SPSS v25 for Windows version 10, and values with p <
0.05 were considered statistically significant.
Results
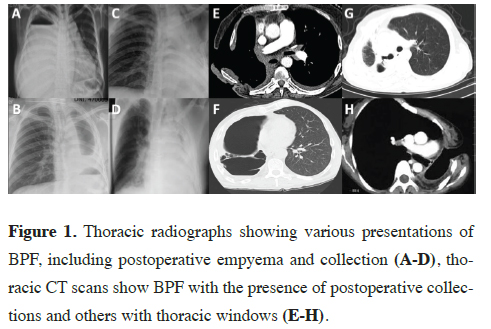
Fifty-seven patients who underwent pneumonectomy were evaluated, 28.1% of whom presented BPF. The mean age of the patients with BPF was 38.3 ± 15 years and the male sex prevailed over the female sex (62.5% vs. 37.5%). Twenty-five percent of the patients with BPF had a history of smoking and alcoholism, with no statistically significant relationship (Figure 1).
 Click Here to Zoom |
Figure 1: Thoracic radiographs showing various presentations of BPF, including postoperative empyema and collection (A-D), thoracic CT scans show BPF with the presence of postoperative collections and others with thoracic windows (E-H). |
About the clinical comparison between the pneumonectomies sample with and without BPF, there was a history of pulmonary tuberculosis in 68.2% vs. 62% (p = 0.225), respectively; however, for patients with BPF, 62.5% had a post-TB fibrocavitary sequela, 18.8% had pulmonary hydatidosis, and 12.5% had a post-surgical cavitary sequela. The mortality associated with BPF was 6.25%, and in the non-BPF group it was 4.87% (p = 0.098). There was malnutrition in 12.5% vs. 2.4% (p = 0.187), both for patients with and without BPF, anemia 51.2% vs.75% (p = 0.225), hypoalbuminemia 31.3% vs. 14.6% (p = 0.260), Diabetes mellitus 18.8% vs. 7.3% (p = 0.335), predicted low FEV1 56.3% vs. 31.7% (p = 0.046), ASA >II 18.8% vs. 9.8% (p = 0.349), mechanical ventilation >24 hours 6.3% vs. 2.4% (p = 0.486), preoperative pleural empyema 37.5% vs. 2.4% (p = 0.01), preoperative neoadjuvant therapy (100% vs. 100%), postoperative pulmonary infection 18.8% vs. 9.8% and postoperative empyema 100% vs. 9.8% (p = 0.00), respectively for both groups (Tables 1,2). The positive bronchial margins for carcinoma with BPF was 12.5%, and in the non-BPF group it was 7.31% (p = 0.012). Regarding the surgical comparison between the pneumonectomies sample with and without BPF, left pneumonectomy predominated in both groups 81.3% vs. 65.9% (p = 0.342), the main suture thread was polypropylene 56.3% vs. 58.5% (p = 0.430), the main bronchial manual closure technique was SWEET 81.3% vs. 97. 6% followed by MABIT 18.8% vs. 2.4% (p = 0.063), for bronchial stump used pleural coverage 56.3% vs. 75.6% (p = 0.199), lymph node dissection 6.3% vs. 2.4% (p = 0.486), emergency surgery 87.5% vs. 82.9% (p = 1.00), operative time 450.69 min. vs. 367.73 min. (p = 0.032) and finally intraoperative bleeding 2220.63 ml. vs. 1579.15 ml. (p = 0.145), respectively for both groups (Tables 1,2).
Table 1: Baseline characteristics of patients who underwent pneumonectomy with and without BF.
Discussion
BPF is a complication that represents 59% of morbidity and 5.4-71% of mortality in patients who underwent pneumonectomy [1-3]. Our research reported an incidence of 28.1% of BPF, with a higher percentage reported in studies by Mammana (4.5%), Gursoy (10.8%), and La Serna (11%) [6,12,13]. We described a history of sequelae of TB infection in 68.4%; however, several authors have reported higher rates exceeding 75% [10,12,14,15]. This could be explained by the fact that chronic inflammation due to tuberculosis leads to the deposition of collagen at the hilar level and stiffer anatomical structures; consequently, bronchial tissue is predisposed to impaired healing and a greater risk of BPF [6]. BPF was found to be mainly associated with the male gender (62.5%); being similar to that reported by La Serna, who described that 69% of the population was male [13]. Mammana et al suggested that a possible explanation could be that the diameter of the male bronchus is larger and when closing the bronchial stump this would generate greater tension; on the other hand, Nachira et al reported that the female sex was an associated factor for post-pneumonectomy BPF (p = 0.03) [3,6]. The mean age of patients with BPF reported in our study (38.31 ± 15 years) was somewhat different from that reported by Fuso and Ahmet et al as they described 66.1 ± 7.4 years and the latter would be due to the etiology associated with BPF such as neoplasms, unlike our case, mainly young population and with infectious etiologies [9,10,16].We identified a statistically significant association (p = 0.015) between smoking and BPF, similar to that reported by Thomas et al and describing that active smoking was a significant predictor of BPF (OR 1.6; 95%CI, 1.34-1.95, p = 0.017) [11]; however, Yazgan and Mazzela et al did not find a clear significant association [8,10]. Likewise, alcoholism was significantly associated with BPF (p= 0.008), similar to that described by Thomas et al (OR 2.2; 95%CI, 1.98-2.45, p = 0.017), describing alcohol abuse and dependence as a negative predictor for BPF [11]. In Peru, the main indication for pulmonary surgery is for sequelae pulmonary TB, and we report more than 50% incidence of pulmonary TB for both groups; however, without statistically significant association with BPF [14]. Mammana et al also reported no significant association in 20% of their cases [6]; but Gursoy and Ahmet et al reported that 33.3% of patients with pulmonary TB were associated with the presence of post-pneumonectomy BPF (p = 0.08) [12,15].
Among the predictors of BPF development described by Pforr et al the presence of non-malignant pulmonary disease (p = 0.03) [10] and among which pulmonary sequelae disease (78%) and hydatidosis (34%), were also described by Birdas, and Ahmet et al [15,17]. Factors such as malnutrition, underweight, anemia, and hypoalbuminemia have been widely related to BPF and were described by Thomas, Pforr, Haraguchi, and Mazzella et al. However, the latter highlighted that hypoalbuminemia was present in more than 20% of patients affected by BPF (p = 0.02) [8,10,11,18]. COPD and low FEV1 predicted postpneumonectomy have been related to the incidence of BPF, because chronic inflammation of the bronchial mucosa in these patients may contribute to impair healing after lung resection [2]. Algar et al described that low postoperative FEV1 is a predictive factor for postpneumonectomy BPF (p = 0.012); however, Yazgan and Thomas et al reported that there is no relationship and that more evidence is needed to accept this [2,11,19]. Nachira (p < 0.019) and Mammana et al (p < 0.001), reported that prolonged mechanical ventilation causes a higher risk of suture tension and has been associated with the presence of BPF [3,6]; however, we do not report a significant association in this item but we do describe that preoperative empyema is strongly associated with this complication and was described by Haraguchi (p = 0.002), stating that preoperative pulmonary infections contribute significantly to BPF in postoperative pneumonectomy patients (p = 0.002) [19], stating that preoperative pulmonary infections contribute significantly to BPF in postoperative pneumonectomy patients [19]. We did not find an association between BPF and postoperative pulmonary infection as did Fuso et al [9]. However, Nachira et al concluded that postoperative pulmonary infections were strong predictors of BPF development (p = 0.008) [3]. Despite this, it has been described that a mechanism of production of BPF is the infection of the pleural space and the increasing accumulation of infected fluid, resulting in the evacuation of empyema through the bronchial stump and subsequent complication into BPF [20]. That is why we report that 100% of the patients who presented BPF had postoperative pleural empyema (p = 0.000), similar to what was described by Nachira et al, who reported that postoperative empyema increases the risk of having BPF by 8 times (p < 0.001) [3] and also Somocurcio et al concluded that postoperative complications in patients who underwent surgery for multidrug-resistant pulmonary TB were postoperative empyema followed by BPF [13]. The greater frequency of BPF in right pneumonectomy is due to its blood vascularization which is nourished by one artery, compared to the left bronchus which is perfused by two bronchial arteries; likewise, another protective mechanism would be that in left pneumonectomy, the bronchial stump is covered by the aorta and surrounding tissues [2,18]. All the above described has been reflected in publications by Mammana (OR 4.08; 95%CI, 3.67-4.67, p = 0.004), Gursoy (OR 5.90; 95%CI, 4.97-6.32, p = 0.001) and Mazzella et al (OR 2.53; 95%CI, 2.11-3.35, p = 0.023), concluding that right pneumonectomy is an associated factor for the development of BPF [6,8,12]. One of the possible causes of BPF is the use of inadequate suture material. Non-absorbable thread (stainless steel, silk, polypropylene) gives better results, the other types are eliminated with the risk of BPF [21]. In the present study; only suture thread was used for bronchial stump closure, no mechanical sutures were used. The most frequent thread used in patients with BPF was polypropylene (56.3%) but no significant association was found. Gursoy et al reported that of the cohort of patients with BPF, automatic suture (74.5%) and manual suture (polypropylene, 25.5%) were used but without significant differences [12]; however, Mammana et al described that the use of vicryl was one of the factors that showed a significant positive trend over time towards BPF (p < 0.001) [6]. Ahmet et al reported that of the mechanical and manual suture group (polypropylene), 5% and 1.5% respectively, developed BPF, and it was concluded that mechanical suture was associated with BPF (p = 0.04) [15]. The bronchus closure technique with continuous stitches (Mabit Technique) was performed in intraoperative complications such as bleeding, which required quick control of the pulmonary hilum, in sequelae lung, chronic empyema, and in case of previous pulmonary resection, where it was very difficult to separate vascular structures and bronchus. With this technique, 75% BPF was described, in contrast to the sweet technique with 32.5%. This factor was evaluated because, with continuous suturing, one of the basic principles of bronchial stump closure would be affected, that of conserving the blood supply and thus affecting the irrigation of the bronchial stump [1]. In the literature, manual and mechanical closure techniques are compared, and their use in bronchial stump closure is still controversial [3,6,9].
Lymph node dissection in pneumonectomy, especially in right pneumonectomy, has been associated with greater devascularization of the bronchial stump, causing greater vascular damage of the nutrient vessel in surgery and being a risk factor for the presence of BPF [2,18], we did not report a significant association due to the few cases; neither was it described in other studies such as those of Pforr, and Thomas et al [10,11]. Mammana et al reported that residual tumor in the bronchial stump is a risk factor for post-pneumonectomy BPF (p = 0.018); however, we did not find any association in the sample studied [6]. La Serna described that the distorted anatomy of patients with sequelae of pulmonary tuberculosis would cause greater intraoperative complications since pulmonary dissections are difficult and increase the operative time [13]. We described a longer operative time in patients who underwent pneumonectomy and BPF than those who did not present this complication, being a significant and related finding (p = 0.032); likewise, Thomas et al also concluded that increased operative time is an associated predictor factor for BPF [11].
Among the limitations of our study was the small sample size of our population; however, despite being one of the leading institutions in the surgical management of TB, we have a small caseload. We also believe that a longerterm follow-up study would be ideal for this group of patients with BPF who underwent pneumonectomy
In conclusion, we have found a series of factors associated with the presence of BPF in patients who underwent pneumonectomy, many of them related to pulmonary tuberculosis infection, which is an important predictor of the associated complications; however, we believe that further multicenter studies with a larger number of patients are needed to identify more factors to be considered during the surgical management of this terrible complication such as BPF.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect
to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research
and/or authorship of this article.
Ethics approval
The study protocol was approved by the ethics committee
of the Universidad de San Martín de Porres (USMP-
00456/2022) and the Hospital Nacional Hipólito Unánue
(NIT2309-06/2022), Lima, Peru.
Authors’ contribution
KGP, WSC: Concept, design, definition of intellectual
content, literature search, clinical studies, experimental
studies, data acquisition, data analysis, statistical analysis,
manuscript preparation, manuscript editing and
manuscript review, PVZ: Data acquisition, data analysis,
manuscript editing and manuscript review.
Reference
1) Wright CD, Wain JC, Mathisen DJ, Grillo HC. Postpneumonectomy
bronchopleural fistula after sutured bronchial closure:
incidence, risk factors, and management. J Thorac Cardiovasc
Surg 1996; 112: 1367-71.
2) Algar FJ, Alvarez A, Aranda JL, Salvatierra A, Baamonde C,
López-Pujol FJ. Prediction of early bronchopleural fistula after
pneumonectomy: a multivariate analysis. Ann Thorac Surg
2001; 72: 1662-7.
3) Nachira D, Chiappetta M, Fuso L, Varone F, Leli I, Congedo
MT et al. Analysis of risk factors in the development of bronchopleural
fistula after major anatomic lung resection: experience
of a single centre. ANZ J Surg 2018; 88: 322-326.
4) Lugo-Álvarez G, Céspedes-Meneses E, Ruiz-Flores J, Téllez-
Becerra J. Fístula broncopleural: tratamiento endoscó-pico con nitrato
de plata. Experiencia de 5 a˜nos en el Instituto Nacional de Enfermedades
Respiratorias. Rev Sanid Milit Mex 2009;63: 178-81.
5) Pomerantz M, Madsen L, Goble M, Iseman M. Surgical management
of resistant mycobacterial tuberculosis and other mycobacterial
pulmonary infections. Ann Thorac Surg 1991; 52: 1108-11.
6) Mammana M, Marulli G, Zuin A, Perissinotto E, Comacchio
GM, De Franceschi E, Rea F. Postpneumonectomy bronchopleural
fistula: analysis of risk factors and the role of bronchial
stump coverage. Surg Today 2020; 50: 114-122.
7) La Serna M. Bronchopleural fistula in pulmonary tuberculosis
surgery at Hospital Nacional Arzobispo Loayza. 2010 - 2015.
University of San Martín de Porres Repository. 2016.
8) JC Sanchez-Hernandez. Bronchopleural fistula as a late complication
of pneumonectomy. J Gen Fam Med 2012; 3: 2.
9) Mazzella A, Pardolesi A, Maisonneuve P, Petrella F, Galetta D, Gasparri
R, Spaggiari L. Bronchopleural Fistula After Pneumonectomy:
Risk Factors and Management, Focusing on Open-Window Thoracostomy.
Semin Thorac Cardiovasc Surg 2018; 30: 104-13.
10) Fuso L, Varone F, Nachira D, Leli I, Salimbene I, Congedo MT,
Margaritora S, Granone P. Incidence and Management of Post-
Lobectomy and Pneumonectomy Bronchopleural Fistula. Lung
2016; 194: 299-305.
11) Pforr A, Pagès PB, Baste JM, Thomas P, Falcoz PE, Lepimpec
Barthes F et al; Epithor Project French Society of Thoracic and
Cardiovascular Surgery. A Predictive Score for Bronchopleural
Fistula Established Using the French Database Epithor. Ann
Thorac Surg 2016; 101: 287-93.
12) Gursoy S, Yazgan S, Ucvet A, Samancilar O, Unal M, Gulmez
B, Sirzai EY. Postpneumonectomy bronchopleural fistula in
non-small cell lung cancer patients: incidence, survival, mortality,
and treatment analysis. Surg Today 2018; 48: 695-702.
13) Somocurcio JG, Sotomayor A, Shin S, Valcárcel M, Portilla S,
Guerra D et al. Surgical treatment of multidrug-resistant pulmonary
tuberculosis in Peru: a series of 304 cases. Jor Peru
Med Exp Public Health 2009; 26: 3.
14) Visbal A, Sánchez R. Transsternal transpericardial approach
for closure of late bronchopleural fistula and management of
chronic empyema associated with tuberculosis. Rev Colomb
Cir 2006; 21: 87-96.
15) Birdas TJ, Morad MH, Okereke IC, Rieger KM, Kruter LE,
Mathur PN, Kesler KA. Risk factors for bronchopleural fistula
after right pneumonectomy: does eliminating the stump diverticulum
provide protection? Ann Surg Oncol 2012; 19: 1336-42.
16) Marcelo J, Sánchez F, Estors M. Main bronchus fistula closure
after pneumonectomy with Figulla Flex Occlutech ASD device.
Archivo De Bronconeumología 2012; 48: 137-8.
17) Samancilar O, SeydaOrs K, Ozan U, Taner O. Neoadjuvant
chemotherapy is a risk factor for bronchopleural fistula after
pneumonectomy for non-small cell lung cáncer. Kardiochir
Torakochirurgia Pol 2014; 11: 40-3.
18) Ahmet U, Soner G, Serdar S, Erbaycu A, Ozturk A, Kenan C
et al. Bronchial closure methods and risks for bronchopleural
fistula in pulmonary resections: how a surgeon may choose the
optimum method? Interact CardioVasc Thorac 2011; 12: 558-62.
19) Djouani A, Hurley P, Lampridis S, Bille A. Successful Closure
of Post-pneumonectomy Bronchopleural Fistula With Suture
Repair Reinforced With Porcine Acellular Dermal Matrix (Permacol)
and Hydrogel Sealant (Progel): A Case Report. Cureus
2022; 14: e28529.
20) Di Maio M, Perrone F, Deschamps C, Rocco G. A meta-analysis
of the impact of bronchial stump coverage on the risk of
bronchopleural fistula after pneumonectomy. Eur J Cardiothorac
Surg 2015; 48: 196-200.
21) Zakkar M, Kanagasabay R, Hunt I. No evidence that manual
closure of the bronchial stump has a lower failure rate than mechanical
stapler closure following anatomical lung resection.
Interact Cardiovasc Thorac Surg 2014; 18: 488-93.
1) Wright CD, Wain JC, Mathisen DJ, Grillo HC. Postpneumonectomy
bronchopleural fistula after sutured bronchial closure:
incidence, risk factors, and management. J Thorac Cardiovasc
Surg 1996; 112: 1367-71.
2) Algar FJ, Alvarez A, Aranda JL, Salvatierra A, Baamonde C,
López-Pujol FJ. Prediction of early bronchopleural fistula after
pneumonectomy: a multivariate analysis. Ann Thorac Surg
2001; 72: 1662-7.
3) Nachira D, Chiappetta M, Fuso L, Varone F, Leli I, Congedo
MT et al. Analysis of risk factors in the development of bronchopleural
fistula after major anatomic lung resection: experience
of a single centre. ANZ J Surg 2018; 88: 322-326.
4) Lugo-Álvarez G, Céspedes-Meneses E, Ruiz-Flores J, Téllez-
Becerra J. Fístula broncopleural: tratamiento endoscó-pico con nitrato
de plata. Experiencia de 5 a˜nos en el Instituto Nacional de Enfermedades
Respiratorias. Rev Sanid Milit Mex 2009;63: 178-81.
5) Pomerantz M, Madsen L, Goble M, Iseman M. Surgical management
of resistant mycobacterial tuberculosis and other mycobacterial
pulmonary infections. Ann Thorac Surg 1991; 52: 1108-11.
6) Mammana M, Marulli G, Zuin A, Perissinotto E, Comacchio
GM, De Franceschi E, Rea F. Postpneumonectomy bronchopleural
fistula: analysis of risk factors and the role of bronchial
stump coverage. Surg Today 2020; 50: 114-122.
7) La Serna M. Bronchopleural fistula in pulmonary tuberculosis
surgery at Hospital Nacional Arzobispo Loayza. 2010 - 2015.
University of San Martín de Porres Repository. 2016.
8) JC Sanchez-Hernandez. Bronchopleural fistula as a late complication
of pneumonectomy. J Gen Fam Med 2012; 3: 2.
9) Mazzella A, Pardolesi A, Maisonneuve P, Petrella F, Galetta D, Gasparri
R, Spaggiari L. Bronchopleural Fistula After Pneumonectomy:
Risk Factors and Management, Focusing on Open-Window Thoracostomy.
Semin Thorac Cardiovasc Surg 2018; 30: 104-13.
10) Fuso L, Varone F, Nachira D, Leli I, Salimbene I, Congedo MT,
Margaritora S, Granone P. Incidence and Management of Post-
Lobectomy and Pneumonectomy Bronchopleural Fistula. Lung
2016; 194: 299-305.
11) Pforr A, Pagès PB, Baste JM, Thomas P, Falcoz PE, Lepimpec
Barthes F et al; Epithor Project French Society of Thoracic and
Cardiovascular Surgery. A Predictive Score for Bronchopleural
Fistula Established Using the French Database Epithor. Ann
Thorac Surg 2016; 101: 287-93.
12) Gursoy S, Yazgan S, Ucvet A, Samancilar O, Unal M, Gulmez
B, Sirzai EY. Postpneumonectomy bronchopleural fistula in
non-small cell lung cancer patients: incidence, survival, mortality,
and treatment analysis. Surg Today 2018; 48: 695-702.
13) Somocurcio JG, Sotomayor A, Shin S, Valcárcel M, Portilla S,
Guerra D et al. Surgical treatment of multidrug-resistant pulmonary
tuberculosis in Peru: a series of 304 cases. Jor Peru
Med Exp Public Health 2009; 26: 3.
14) Visbal A, Sánchez R. Transsternal transpericardial approach
for closure of late bronchopleural fistula and management of
chronic empyema associated with tuberculosis. Rev Colomb
Cir 2006; 21: 87-96.
15) Birdas TJ, Morad MH, Okereke IC, Rieger KM, Kruter LE,
Mathur PN, Kesler KA. Risk factors for bronchopleural fistula
after right pneumonectomy: does eliminating the stump diverticulum
provide protection? Ann Surg Oncol 2012; 19: 1336-42.
16) Marcelo J, Sánchez F, Estors M. Main bronchus fistula closure
after pneumonectomy with Figulla Flex Occlutech ASD device.
Archivo De Bronconeumología 2012; 48: 137-8.
17) Samancilar O, SeydaOrs K, Ozan U, Taner O. Neoadjuvant
chemotherapy is a risk factor for bronchopleural fistula after
pneumonectomy for non-small cell lung cáncer. Kardiochir
Torakochirurgia Pol 2014; 11: 40-3.
18) Ahmet U, Soner G, Serdar S, Erbaycu A, Ozturk A, Kenan C
et al. Bronchial closure methods and risks for bronchopleural
fistula in pulmonary resections: how a surgeon may choose the
optimum method? Interact CardioVasc Thorac 2011; 12: 558-62.
19) Djouani A, Hurley P, Lampridis S, Bille A. Successful Closure
of Post-pneumonectomy Bronchopleural Fistula With Suture
Repair Reinforced With Porcine Acellular Dermal Matrix (Permacol)
and Hydrogel Sealant (Progel): A Case Report. Cureus
2022; 14: e28529.



