

Summary
Background: Chylothorax is defined as the accumulation of chyle in the pleural space. In this report, we discuss the demographic features of patients with post-operative chylothorax, and the treatment approaches used to emphasize the importance of this complication for thoracic surgery.Materials and Methods: Medical records of 13 patients operated between January 2001 and July 2017, and who were diagnosed with chylothorax in the post-operative period, were retrospectively evaluated. Patients" demographic features, type of surgeries, results of histopathological examination, day of chylothorax diagnosis, and treatment results were recorded.
Results: Eight male patients (61.5%) and 5 female patients (38.5%) were included in the study. Chylothorax developed after lobectomy in 9 patients (62.23%), total pleurectomy in 2 patients (15.38%), bilobectomy in 1 patient (7.69%), and pneumonectomy in 1 patient (7.69%). Oral nutrition was discontinued immediately after chylothorax diagnosis, and patients were followed-up with parenteral nutrition. A conservative approach was used in 11 patients (84.61%). Two patients (15.39%) did not respond to medical treatment, and underwent surgery. All patients were successfully treated chylothorax.
Conclusions: Chylothorax is a rare but serious post-operative complication after thoracic surgeries. While appropriate conservative treatment leads to recovery in majority of the cases, surgery remains an important option for patients with prolonged drainage and persistent disease.
Introduction
Chylothorax is defined as the accumulation of chyle in the pleural space [1]. The disease etiology consists of various factors, including congenital factors, trauma, infections, and malignancies that disrupt the thoracic lymph flow (e.g. lymphoma) [1,2]. Iatrogenic cases are the leading cause of traumatic chylothorax [3]. Iatrogenic injury to ductus thoracicus commonly occurs during surgical procedures [3]. Post-operative chylothorax is more common after esophagus surgery (0.9-11.6%), and its incidence after pulmonary resection ranges between 1.9-4% [3-12].The first striking symptom of post-operative chylothorax is the drainage of a creamy pleural fluid, which is elevated with the onset of oral nutrition [9-14]. Elevated triglyceride levels in pleural fluid (>110 mg/dL) and a cholesterol/triglyceride ratio <1 are usually sufficient for diagnosis [9-13]. The first step of treatment is to limit oral nutrition, and supplementary treatment [15]. The use of somatostatin analogs has also resulted in favorable outcomes [16,17]. Surgical treatment is still important, while percutaneous embolization techniques are considered as alternatives for treatment of patients who do not respond to medical treatment [18-20]. Here, we discuss the post-operative chylothorax cases in the last 16 years, and the treatment approaches we used. We also discuss the challenges in management of this rare but serious post-operative thoracic surgery complication.
Methods
Medical records of patients, who were diagnosed with chylothorax in the post-operative period between January 2001 and July 2017, were retrospectively evaluated. Written informed consent forms were obtained from all patients. Demographic features, including age, gender, type of surgery, histopathological examination, were recorded. A standard approach was used for diagnosis of all patients. Blood and pleural fluid specimens were collected simultaneously from patients with elevated fluid drainage during post-operative follow-up, and the specimens were sent for biochemical analyses. Chylothorax diagnosis was made when one of the following conditions was satisfied: Having a pleural fluid TG level >110 mg/dl or having a pleural fluid TG level > blood TG level and a pleural fluid cholesterol level/TG level ratio <1. Oral nutrition was discontinued upon chylothorax diagnosis. After consulting with gastroenterology clinic and a nutritionist, TPN was arranged for all patients. One patient underwent surgery in the early post-operative period due to a massive air leak. All other patients were followed up with conservative treatment for chylothorax. Nonresponders (i.e. patients with persistent chylothorax despite medical treatment) were scheduled to undergo surgery.Oral nutrition was started gradually, when the level of pleural fluid drainage decreased after appropriate medical treatment, and when the fluid was serous. The decision for discontinuing drainage was based on the appearance, volume, and biochemical analysis of pleural fluid. Drainage was discontinued when a serous pleural fluid drainage despite a fat-rich diet and a volume <100 cc per day was achieved. Prior to discharge, all patients were evaluated with chest x-rays.
Results
Eight male patients (61.5%) and 5 female patients (38.5%) were included in the study. The mean age of male patients was 64.62 ± 7.22 (53-75) / years, and the mean age of female patients was 55 ± 6.60 (49-67) / years. The mean age of all patients was 60.9 ± 8.41 (49-75) / years. According to the medical records, 5 patients (38.5%) underwent right upper lobectomy, 2 patients (15.3%) underwent left upper lobectomy, 2 patients (15.3%) underwent left lower lobectomy, 2 patients underwent pleura decortication and pleurectomy, 1 patient (7.7%) underwent left pneumonectomy, and 1 patient (7.7%) underwent lower bilobectomy. Histopathological examination showed that 6 patients (46.2%) had primary lung adenocarcinoma, 4 patients (30.8%) had squamous cell carcinoma, and 2 patients (15.3%) had epithelial-type malignant mesothelioma, and 1 patient (7.7%) had bronchiectasis (Table 1).Table 1: Demographic features of patients
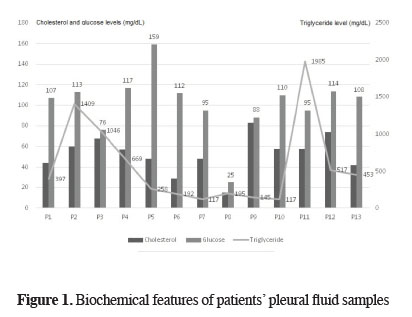
For all patients, chylothorax diagnosis was done after biochemical examination of blood and pleural fluid samples that were taken simultaneously. Biochemical analyses of pleural fluid samples showed a total protein level of 3.12 ± 0.51 (2.10-3.90) g/dL, albumin level of 1.71 ± 0.31 (1.20-2.10) g/dL, glucose level of 101.46 ± 30.08 (25-159) mg/dL, lactate dehydrogenase (LDH) level of 789.38 ± 514.50 (236-1979) U/L, cholesterol level of 52.61 ± 18.14 (15-83) mg/dL (Figure 1), and TG level of 576.92 ± 574.47 (117-1985) mg/dL (Table 2). Eight patients were diagnosed with chylothorax on day 1, 4 patients were diagnosed on day 2, and 1 patient was diagnosed on day 3. The mean time of diagnosis was post-operative 1.46 ± 0.63 (1-3) / days.
 Click Here to Zoom |
Figure 1: Biochemical features of patients" pleural fluid samples |
Table 2: Biochemical parameters in pleural fluid
Oral nutrition was discontinued immediately after chylothorax diagnosis. After consulting with gastroenterology clinic and a nutritionist, TPN was arranged for all patients. One patient was scheduled for surgery on day 4 due to a massive air leak. Air leak control and mass ligation were performed. All other patients were followed up by medical treatment. During follow-up, only 2 patients had persistent chyle drainage for >14 days. One of the patients underwent surgery for mass ligation. The other patient continued to receive medical treatment, and responded to treatment on day 21. After surgery, regression was seen in both patients. Overall, oral nutrition returned to normal on post-operative 9.61 ± 2.98 (6-21) / days. The mean duration of drainage was 13.6 ± 5.72 (7-29) / days, and the mean hospital stay was 20.46 ± 6.61 days (10-33) / days.
Three patients had secondary complications. Two patients had empyema related to prolonged drainage, and 1 patient had candida sepsis secondary to TPN infusion. Appropriate medical treatments were arranged for these 3 patients. Antibiotic treatment was discontinued when three consecutive cultures tested negative, and all patients were discharged after complete recovery. We did not observe mortality in any of the patients.
Discussion
Post-operative chylothorax is a rare and serious complication after thoracic surgery; yet, when untreated, it is associated with high morbidity and mortality rates [9,12]. The prevalence of chylothorax after lung resection differs between previous reports. Cho et al. [7] reported that incidence of chylothorax is 2.1% in patients who undergo pulmonary resection. Shinsuke et al. [1] reported chylothorax in 50 of 1,235 patients (4%) while Kimihiro et al. [14] reported chylothorax in 27 of 1,110 patients (2.4%). Similar findings were also reported by Takuwa et al. [13] in a series of 1,580 patients who underwent surgery due to lung cancer. The authors reported chylothorax development in 37 patients (2.3%), and 36 of these patients underwent lobectomy. Aggressive lymph node dissection has been reported as the cause of iatrogenic chylothorax development [13,14,21]. In the current study, 9 patients (62.23%) underwent lobectomy, 1 patient (7.69%) underwent bilobectomy, 1 patient (7.69%) underwent pneumonectomy, and 2 patients (15.38%) underwent total pleurectomy for mesothelioma. All patients, except one patient who underwent surgery for bronchiectasis, underwent surgery due to different malignancies, and in all cases, lymph node dissection were performed according to oncological principles. In this regard, lymph node dissection is the common operation, and is unlikely to be responsible for chylothorax. Different surgical treatments, including pleurodesis, mass ligation and pleurectomy, can be performed for treatment of chylothorax. Pleurectomy can be the preferred option, especially for pediatric patients with a disease that is difficult to treat [22-24]. Development of chylothorax after a treatment for chylothorax is surprising. This paradox can be explained by the fact that both patients had malignant mesothelioma diagnosis, and underwent aggressive pleurectomy in light of oncological principles. It is possible that ductus thoracicus is damaged during resection of mediastinal pleura.Despite the minor differences in definition of post-operative chylothorax diagnosis, the majority of the studies consider the presence of milky pleural fluid and elevated TG levels in pleural fluid (>110 mg/dL) to be sufficient for diagnosis [8-10]. In the current study, we evaluated blood TG levels, in addition to pleural TG levels, for differential diagnosis of potential hyperlipidemia. An additional condition was to have a cholesterol/TG ratio <1 in pleural fluid.
Chyle has vital importance as it contains proteins, immunoglobulins, electrolytes, essential amino acids, and vitamins; loss of immunoglobulins results in immunosuppression, and chronic loss of chyle causes severe problems, including mortality [2,8,25]. Thus, the treatment of this condition is equally important as its diagnosis. The treatment options in the literature include algorithms that consider the level of daily drainage volume, while other studies suggest a 14-day waiting period before surgery [8,9,16,17,20]. Following chylothorax diagnosis, discontinuation of oral nutrition and replacement of chyle with appropriate parenteral treatment are considered to be the first stage of treatment [8,9,13,14]. Liu et al. [10] evaluated the volume of drainage after 4 days of medical treatment, and considered patients with a drainage volume >400 cc as candidates for surgical treatment. Those in favor of conservative treatment as the first treatment option state that surgical treatment should be chosen when conservative treatment for 14 days fails [8,26,27]. Cerfolio et al. [9] reported post-operative chylothorax development in 47 of 11,315 patients who underwent thoracic surgery. After pulmonary resection, chylothorax developed in 13 patients, and 8 patients recovered with conservative treatment. In the current study, oral nutrition was discontinued, and patients were followed-up with conservative treatment. Only 1 patient underwent surgery on day 4 due to an air leak. In case of 1 patient, surgery was performed as the patient did not respond to medical treatment. In case of 11 patients (84.61%), complete response was observed with only conservative therapy.
Another key point on chylothorax treatment is the use of somatostatin analogs. The combination of somatostatin analogs and conservative treatment has produced successful results [16,17]. In the current study, somatostatin analogs were used in only 2 patients; one of the patients underwent surgery after a 14-day waiting period. For the other patient, we did not determine a significant difference in the ending of chyle leak.
Overall, chylothorax was successfully treated with medical treatment in 11 of 13 patients, and our results are consistent with the literature. Liu et al. [10] reported chylothorax development in 20 of 776 patients (2.58%) who underwent pulmonary resection and mediastinal lymph node dissection for non-small cell lung carcinoma. In this study, 75% of the patients received conservative treatment, and 25% underwent another surgery due to chylothorax. The authors reported that the mean length of hospital stay is 19.3 days. Takuwa et al. [13] reported that 84% of patients recovered after conservative treatment. Shimizu et al. [14] showed that 81% of the patients are recovered after conservative therapy, whereas Bryant et al. [28] reported that 90% of the patients are treated with medium-chain triglyceride diet and somatostatin.
The need to perform additional procedures arises when patients do not respond to medical treatment (i.e. cases of persistent post-operative chylothorax). Embolization of thoracic duct with percutaneous methods has been suggested as an alternative revision surgery [11,18]. Surgery becomes inevitable when interventional approaches fail. Another crucial point is to locate the site of the leak. Bommart et al. [11] suggested that lipiodol lympography is a useful method to determine the leak. Alternative methods include evans blue, sudan III dye, and ingesting food (high fat or cream) mixed with sudan black dye [9,19]. In the current study, we did not use any additional method to locate the leaks. The same surgical team was responsible for revision surgeries in both early and late periods. Chyle leak was closed in both patients after operation.
Loss of immunoglobulin loss results in immunosuppression, and predisposes patients to a higher risk of opportunistic infections [8]. In the current study, empyema was detected during medical treatment of 2 patients. In another patient, candida sepsis developed secondary to parenteral nutrition. Overall, the treatment of post-operative chylothorax is a challenging process for both patients and surgeons, and requires patience. While medical treatment usually yields successful outcomes, surgical treatment may be required in certain cases. It should be kept in mind that prolonged parenteral nutrition and drainage can lead to secondary complications.
Declaration of conflicting interests
The author declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The author received no financial support.
Reference
1) DeMeester T. The pleura. In: Spencer E, Editor. Surgery of the chest. 4th ed., Philadelphia: WB Saunders; 1983.
2) Bessone L, Ferguson T, Burford T. Chylothorax: a collective review. Ann Thorac Surg 1971;12: 527-50.
3) McWilliams A, Gabbay E. Chylothorax occurring 23 years post-irradiation: literature review and management strategies. Respirology 2000; 5: 301-3.
4) Dougenis D, Walker WS, Cameron EW, Walbaum PR. Management of chylothorax complicating extensive esophageal resection. Surg Gynecol Obstet 1992; 174: 501-6.
5) Choh CT, Khan OA, Rychlik IJ, McManus K. Does ligation of the thoracic duct during oesophagectomy reduce the incidence of post-operative chylothorax? Int J Surg 2012; 10: 203-5.
6) Cagol M, Ruol A, Castoro C, Alfieri R, Michieletto S, Ancona E. Prophylactic thoracic duct mass ligation prevents chylothorax after transthoracic esophagectomy for cancer. World J Surg 2009; 33: 1684-6.
7) Cho HJ, Kim DK, Lee GD, Sim HJ, Choi SH, Kim HR, et al. Chylothorax complication pulmonary resection for lung cancer: Effective management and pleurodesis. Ann Thorac Surg 2014; 97: 408-13.
8) Nair SK, Petko M, Hayward MP. Aetiology and management of chylothorax in adult. Eur J Cardiothorac Surg 2007; 32: 362-9.
9) Cerfolio RJ, Allen MS, Deschamps C, Trastek VF, Pairolero PC. Postoperative Chylothorax. J Thorac Cardiovasc Surg 1996; 112: 1361-6.
10) Liu CY, Hsu PK, Huang CS, Sun YH, Wu YC, Hsu WH. Chylothorax complicating video-assisted thoracoscopic surgery for non-small cell lung cancer. World J Surg 2014; 38: 2875-81.
11) Bommart S, Berthet JP, Durand G, Pujol JL, Mathieu C, Marty-Ané C, et al. Imaging of postoperative complications following surgery for lung cancer. Diagn Interv Imaging 2017; 98: 11-20.
12) Furukawa M, Tao H, Tanaka T, Okabe K. Mediastinal chyloma after lung cancer surgery: case report. J Cardiothorac Surg 2016; 11: 115.
13) Takuwa T, Yoshida J, Ono S, Hishida T, Nishimura M, Aokage K, et al. Low-fat diet management strategy for chylothorax after pulmonary resection and lymph node dissection for primary lung cancer. J Thorac Cardiovasc Surg 2013; 146: 571-4.
14) Shimizu K, Yoshida J, Nishimura M, Takamochi K, Nakahara R, Nagai K. Treatment strategy for chylothorax after pulmonary resection and lymph node dissection for lung cancer. J Thorac Cardiovasc Surg 2002; 124: 499-502.
15) Valentine VG, Raffin TA. The Management of Chylothorax. Chest 1992; 102: 586-91.
16) Markham K, Glover J, Welsh R, Lucas R, Bendick P. Octreotide in the treatment of thoracic duct injuries. Am Surg 2000; 66: 1165-7.
17) Rimensberger P, Muller-Schenker B, Kalangos A, Beghetti M. Treatment of a persistent postoperative chylothorax with somatostatin. Ann Thorac Surg 1998; 66: 253-4.
18) Itkin M, Kucharczuk JC, Kwak A, Trerotola SO, Kaiser LR. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg 2010; 139: 584-9.
19) Fahimi H, Casselman FP, Mariani MA, van Boven WJ, Knaepen PJ, van Swieten HA. Current management of postoperative chylothorax. Ann Thorac Surg 2001; 71: 448-51.
20) Le Pimpec-Barthes F, D"Attellis N, Dujon A, Legman P, Riquet M. Chylothorax complicating pulmonary resection. Ann Thorac Surg 2002; 73: 1714-9.
21) Uchida S, Suzuki K, Hattori A, Takamochi K, Oh S. Surgical intervention strategy for postoperative chylothorax after lung resection. Surg Today 2016; 46: 197-202.
22) Brunner W. The spontaneous, non-traumatic chylothorax. Therapy by means of pleurectomy and decortication. Schweiz Med Wochenschr 1977; 107: 213-4.
23) Teitelbaum DH, Teich S, Hirschl RB. Successful management of a chylothorax in infancy using a pleurectomy. Pediatr Surg Int 1996; 11: 166-8.
24) Doerr CH, Miller DL, Ryu JH. Chylothorax. Semin Respir Crit Care Med 2001; 22: 617-26.
25) Williams KR, Burford TH. The Management of Chylothorax. Ann Surg 1964; 160: 131-140.
26) Marts B, Naunheim K, Fiore A, Pennington D. Conservative versus surgical management of chylothorax, Am J Surg 1992; 164: 532-4.
27) Selle J, Snyder W, Schreiber J Chylothorax: indications for surgery, Ann Surg 1973; 177: 245-9.



