

2Department of Pulmonary Disease, Haydarpasa Numune Education and Research Hospital, Istanbul, Turkey DOI : 10.26663/cts.2018.00014
Summary
Herpes zoster also known as shingles is a disease caused by the reactivation of varicella zoster virus. It often starts with erythema as the first finding. Maculopapular vesicles may form painful rashes with maturations, which may be painful at baseline before the onset of rash. Here, the postponement of the operation due to the central mass located in the left lobe of the patient due to herpes zoster, and the possible results were presented.Introduction
Herpes zoster, commonly known as shingles, is a disease caused by the reactivation of varicella zoster virus (VZV). Herpes zoster is characterized by an unilateral vesicular rash accompanied by severe neuralgia. It is caused by the reactivation of the varicella zoster virus lying in the sleeping sensory ganglia following the primary infection. The risk of reactivation increases with age and it is estimated that herpes zoster develops in about 50% of the people living up to 85 years [1]. Here, a 76-year-old man who was scheduled for surgery due to squamous cell carcinoma where the surgery was delayed for 3 weeks due to herpes zoster diagnosed in the preoperative period is presented in the light of the relevant literature.Case Presentation
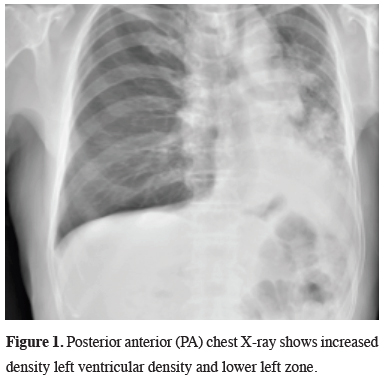
A 74-year-old male patient applied to our clinic with complaints of cough and back pain in the left region. His biography includes hypertension and active smoking. There is no obvious feature in the physical examination. Blood pressure was 135/90 mm/Hg, pulse was 100 beats/min, fever was 36.5 °C and respiratory rate was 24/min. In blood count white blood cell count was 6300/mm3 (68.3% neutrophil and 15% lymphocyte), erythrocyte sedimentation rate was 91 mm/hr, while other laboratory parameters were within normal limits. Posterior anterior (PA) chest X-ray showed increase in density in the left hilar region and in the left lower zone (Figure 1).
 Click Here to Zoom |
Figure 1: Posterior anterior (PA) chest X-ray shows increased density left ventricular density and lower left zone. |
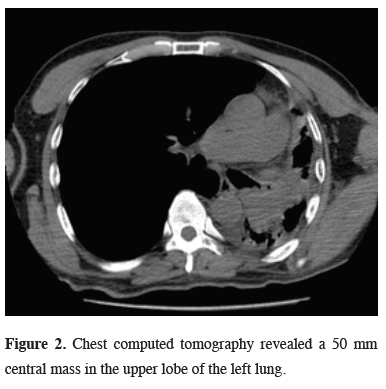
Chest computed tomography (CT) and positron emission tomography (PET/CT) reported a 50 mm central mass (SUVmax: 13) in the left upper lobe of the lung (Figure 2).
 Click Here to Zoom |
Figure 2: Chest computed tomography revealed a 50 mm central mass in the upper lobe of the left lung. |
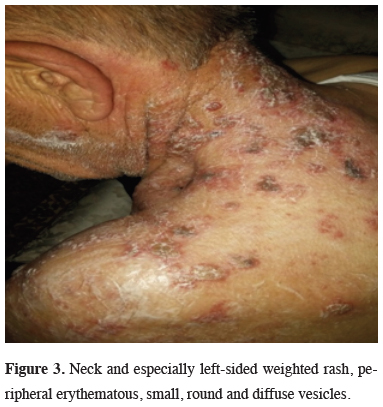
Fiberoptic bronchoscopy (FOB) examination revealed an endobronchial lesion in the left upper lobe bronchus and biopsy was reported as squamous cell carcinoma. Lung function tests were within normal limits (FEV1: 2.1 L). The patient was found to have suspicious mucocutaneous lesions (Figure 3) and no pathology was detected in the biopsy of these lesions. Herpes zoster was diagnosed clinically.
 Click Here to Zoom |
Figure 3: Neck and especially left-sided weighted rash, peripheral erythematous, small, round and diffuse vesicles. |
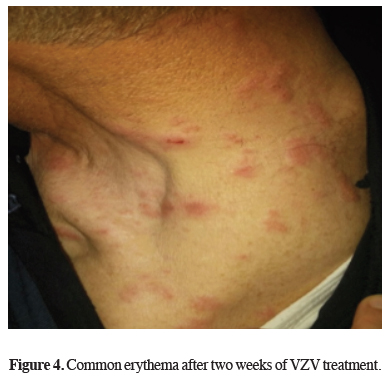
Analgesic and acyclovir treatment lasted for fourteen days. Common erythema after two weeks of VZV treatment is seen in Figure 4.
 Click Here to Zoom |
Figure 4: Common erythema after two weeks of VZV treatment. |
Mediastinoscopy was performed and lymph nodes (2R-4R-7) were primarily sampled. Left pneumonectomy was performed with thoracotomy on preoperative lymph node reactive residence. The case was pathologically staged as T3N0M0 Stage IIB. The patient was discharged uneventfully with no recurrences at 18 month follow-up.
Discussion
Herpes zoster is a contagious disease caused by the reactivation of varicella zoster virus. The virus is often dormant in sensory ganglia of cranial and spinal nerves after chickenpox, but may resume in patients due to advanced age, immunosuppressive medical conditions and malignancies [1]. Epidemiological studies have shown that zoster events have increased in recent years. It is estimated that between 10% and 20% of the world population will suffer life-threatening zoster. There are about 60,000 to 300,000 cases annually in the United States and the majority of this patients are over 50 years old [2]. Risk factors that may increase the likelihood of shingles include immunosuppression, advanced age, some neoplasms and systemic diseases. The reasons behind the reactivation of the VZV are not clear [3]. Lung cancer and VZV findings were detected at the same time in our case of 76 years old.Herpes zoster can usually be diagnosed clinically. However, the diagnosis can be confirmed by the vesicle exacerbations seen with the viral cultures of the vesicular juice, which reveal the multicellular giant cells. Histology reveals inflammation and neuronal loss in the corresponding ganglia in affected dermatomes [4]. In our case, lung cancer and VZV were detected in the same hemithorax region. It shows that VZV may be a secret cancer marker. Evidence for subtype cancers suggests a strong association between herpes zoster and occult hematological cancer. In an anthropological review published in 1995, the relationship between herpes zoster and the diagnosis of subsequent malignancies was examined. Based on only two small basic publications of this review, the authors of the review concluded that there was no increased risk of cancer within herpes zoster patients [5]. Various biological mechanisms explaining the relationship between cancer and herpes zoster has been previously suggested [6]. Findings of VZV in our patient may be related to lung cancer. First, carcinogenesis can induce immunodeficiency, which causes the herpes zoster to burst in the preclinical asymptomatic stage. In particular, it may lead to impaired number and function of B-cells and T-cells positively associated with hematological cancers and these cancers. The risk of hematological cancer increases even 5 to 10 years after the herpes zoster and this immunosuppressive effect indicates that diagnosis of cancer can be detected several years ago [7]. Cellular immune function is firstly important for suppressing varicella-zoster virus replication. Secondly, cellular immune function is crucial to suppress varicella-zoster virus replication and carcinogenesis. For this reason, herpes zoster may be a marker of impaired immune surveillance in the host and in this case causes cancer development. For example, immunosuppression in autoimmune diseases can increase the risk of herpes zoster and some cancers [8]. Thirdly, it is possible that recurrent antigenic stimulation resulting from subclinical and eventual activation of the clinical varicella zoster virus leads to exacerbation of precancerous genetic lesions or triggering neoplastic transformation. Various herpesviruses act directly on tumor-induced disease through inflammation, decreased immunity and immunosuppression. A direct carcinogenic effect of herpes zoster may also explain the cancer development in previous areas affected by herpes zoster [9].
Shingles can occur with local trauma or procedures. The majority of the reported cases develop in the same dermatome as the previous trauma. However, several cases have been reported about where the rashes are located in the real incision area. These cases show that shingles events developed around an incision site may mimic other rash causes. There were very few reports on the surgical incisions of the patients that developed shingles. While Godfrey et al reported that the thoracic surgeon for scoliosis was a zona-developing disease at the surgical incision site, Choi et al published a case report of a patient who developed a herpes zoster within the last face surgery scar [10]. Thomas et al. published a case-control study showing that the risk of shingles increased for one month following trauma, 39 studies in this study identified an increased risk for breast cancer during the next two years of radiotherapy [11]. However, Gadient et al. studied the time interval between the process and the development of the rash [12]. The purpose of treating shingles is to reduce the duration of symptoms of the disease, reduce the risk of transmission and prevent post-herpetic neuralgia. Post-herpetic neuralgia is a syndrome that develops in about 10% to 15% of cutaneous hypersensitivity and neuropathic pain and is particularly difficult to treat [13]. Our case was operated after VZV treatment.
Only a few studies have examined the interactions with age and time after the diagnosis of malignancy [14]. As a conclusion, the relationship between herpes zoster and malignancies should be kept in mind.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Reference
1) Arvin A. Aging, immunity, and the varicella-zoster virus. N Engl J Med 2005; 352: 2266-7.
2) Schmader K, George LK, Burchett BM, et al. Racial differences in the occurrence of herpes zoster. J Infect Dis 1995; 171: 701-4.
3) Watson CP. Postherpetic neuralgia: the importance of preventing this intractable end-stage disorder. J Infect Dis 1998; 178: S91-4.
4) Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med 2000; 342: 635-45.
5) Smith JB, Fenske NA. Herpes zoster and internal malignancy. South Med J 1995; 88: 1089-92.
6) Cotton SJ, Belcher J, Rose P, K Jagadeesan S, Neal RD. The risk of a subsequent cancer diagnosis after herpes zoster infection: primary care database study. Br J Cancer 2013; 108: 721-6.
7) Rawstron AC, Bennett FL, O’Connor SJM, Kwok M, Fenton JAL, Plummer M, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med 2008; 359: 575e83.
8) Forbes HJ, Bhaskaran K, Thomas SL, Smeeth L, Clayton T, Langan SM. Quantification of risk factors for herpes zoster: population based case-control study. BMJ 2014; 348: g2911.
9) Alibek K, Baiken Y, Kakpenova A, Mussabekova A, Zhussupbekova S, Akan M, et al. Implication of human herpesviruses in oncogenesis through immune evasion and suppression. Infect Agents Cancer 2014; 9: 3.
10) Choi HJ, Kim JH, Lee YM. Herpes zoster developing within recent subciliary incision scar. J Craniofac Surg 2012; 23: 930-1.
11) Gadient PM, Smith JH, Ryan SJ. Herpes zoster ophthalmicus following on abotulinumtoxin A administration for chronic migraine: A case report and literature review. Cephalalgia 2015; 35: 443-8.
12) Choo PW, Galil K, Donahue JG, Walker AM, Spiegelman D, Platt R. Risk factors for postherpetic neuralgia. Arch Intern Med 1997; 157: 1217-24.



