

2Department of Thoracic Surgery, Gazi University, School of Medicine, Ankara, Turkey DOI : 10.26663/cts.2019.0005
Summary
Background: Our objective in this study was to prospectively evaluate the effects of conventional closure techniques, intracostal suture, and intercostal nerve preservation on postoperative morbidity and longterm pain management.Materials and Methods: This prospective study was conducted between 2012 and 2015. Three different closure techniques were applied to patients who underwent posterolateral thoracotomy: conventional suture technique, intracostal suture technique, and intracostal suture technique with intercostal nerve preservation. Early postoperative pain level was assessed using a visual analog scale; pain at postoperative six months was assessed with the LANNS pain scale.
Results: Seventy-three male patients (82%) and 16 (18%) female patients were included in the study. Suturing time differed significantly between the groups. At postoperative day 0, 1, and 2, there were significant differences between the groups in pain scores when at rest and while coughing (p < 0.001). Complications occurred in 22 patients (24.7%). There were no significant differences in complications between the groups (p = 0.603). Chronic pain at postoperative six months was more prevalent in group A compared to groups B and C (p < 0.05).
Conclusion: Compared to the conventional technique, the intracostal suture technique with intercostal muscle flap is yields better outcomes in terms of early and chronic postoperative pain.
Introduction
Pain is the main problem affecting patients’ treatment adherence and daily activities after surgery. Thoracotomy is considered one of the most painful of all surgical procedures [1]. Although there are many studies in the literature on this subject, there is still no clear treatment method [2]. Severe pain in the early postoperative period increases the rate of complications [3]. Early complications include atelectasis, pneumonia, and respiratory failure, while a chronic pain in the late postoperative period impacts patients’ daily lives [4,5]. Chronic pain, which is defined as pain that persists for more than six months, occurs at a rate of 67% [2,6].Our objective in this study was to prospectively assess the effects of conventional closure techniques and intracostal and intercostal nerve preservation on postoperative morbidity and long-term pain management.
Methods
This prospective study was conducted between April 2012 and November 2015. The study was approved by the institutional review board and was conducted in accordance with the principles of the Declaration of Helsinki (Version: B.10.4.ISM.4.06.68.48/184). The preoperative informed consent form was taken from all patients.All patients in the study underwent posterolateral thoracotomy due to benign and malignant diseases. Of the 126 patients evaluated for the study, 89 patients were included. The patient flow chart for the study is shown in Figure 1.
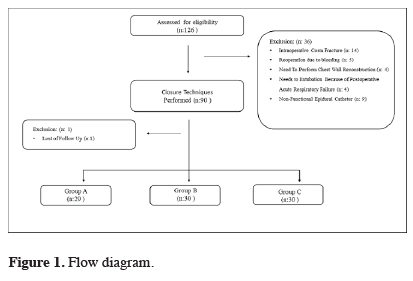 Click Here to Zoom |
Figure 1: Flow diagram. |
Surgeries for malignant disease included lobectomy, pneumonectomy, segmentectomy, and metastasectomy; surgeries for benign disease included lobectomies (bronchiectasis, destroyed lung, and aspergillomas), wedge resection (diagnostic operations), and others (diaphragmatic eventration, decortication, paracardiac cyst).
The patients’ comorbidities were evaluated in three groups: cardiac problems (past myocardial infarction, heart failure, and arrhythmia), respiratory problems (chronic obstructive pulmonary disease, asthma), and endocrine disorders (diabetes mellitus, goiter).The same surgical team performed all procedures. The patients and observers were blinded to the randomization.
Exclusion criteria for the study included age <18 years; history of previous surgery (thoracotomy); undergoing surgery involving thoracic wall resection; pre-perioperative rib fracture; reoperation due to bleeding; not placed epidural catheter (preoperative epidural catheter wasn’t inserted or postoperative catheter displaced early period), needs to intubation because of postoperative acute respiratory failure.
Surgical Technique
For all patients, an epidural catheter was inserted in the area of the fourth or fifth thoracic vertebra under local anesthesia with no intraoperative sedation. With this method, 0.2 g bupivacaine and 500 mg fentanyl were administered postoperatively in 100 cm3 of saline infused at four cm3/s. The epidural catheter was removed on postoperative day 3.
Posterolateral thoracotomy (preserving the serratus anterior muscle) was performed in all procedures. Incisions measuring approximately 20-30 cm were made in the fifth or sixth intercostal space. Ribs were not removed or cut during the procedures.
Three different surgical techniques were used in the study. In group A (conventional suture technique), a Finochietto retractor was placed in the intercostal space during the procedure. At the end of the procedure, two 1/0 polyglactin (Vicryl) sutures were passed through the intercostal muscles to close the intercostal space (Figure 2a). In group B (intracostal suture technique), a Finochietto retractor was placed in the intercostal space during the procedure, as in group A. After the operation; four holes were drilled in the anterior and posterior of the fifth and sixth ribs. In the first ten patients, the holes were made with a hand-held surgical drill. Later, a sternum drill was used to shorten procedure time. Two 1/0 polyglactin (Vicryl) sutures were passed through the holes to close the intercostal space. In group C, the intracostal muscle (ICM) was partially dissected. The Finochietto retractor was placed in the gap created with this dissection. At the end of the procedure, four holes were drilled in the fifth and sixth ribs, and 1/0 polyglactin (Vicryl) sutures were passed over the ICM to close the intercostal space (Figure 2c). A 28 French thoracic tube was placed in all patients after the procedure.
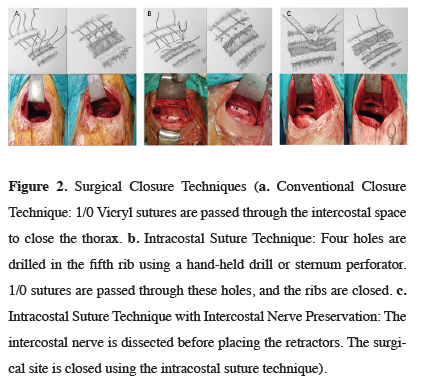 Click Here to Zoom |
Figure 2: Surgical Closure Techniques (a. Conventional Closure Technique: 1/0 Vicryl sutures are passed through the intercostal space to close the thorax. b. Intracostal Suture Technique: Four holes are drilled in the fifth rib using a hand-held drill or sternum perforator. 1/0 sutures are passed through these holes, and the ribs are closed. c. Intracostal Suture Technique with Intercostal Nerve Preservation: The intercostal nerve is dissected before placing the retractors. The surgical site is closed using the intracostal suture technique). |
Postoperative Follow-up
Early postoperative monitoring was done in the intensive care unit. After their general conditions stabilized, the patients were monitored in the inpatient unit. In addition to epidural analgesia, the patients were also administered pethidine 50 mg im 4 times a day on the postoperatif day along with diclofenac potassium 2 x 50 mg po and paracetamol 3 x 500 mg po.
In the early postoperative period, the pain was evaluated using a visual analog scale (VAS) in which a score of 0 indicated no pain and a score of 10 indicated the worst pain ever experienced. Pain monitoring was done every 4 hours from postoperative hour 0 to hour 72. Pain levels while at rest and while coughing were assessed.
LANSS (Leeds Assessment of Neuropathic Symptoms and Signs) pain scoring and VAS assessment were repeated at postoperative six months to evaluate chronic neuropathic pain. The LANSS scale is evaluated out of a total of 24 points. Patients with a score of 12 points or less were considered as not having neuropathic pain, while patients with a score higher than 12 points evaluated as having neuropathic pain. VAS assessment at six months was done in the same way as the previous assessments [7,8].
Postoperative complications that occurred while patients were in the hospital were evaluated. Arrhythmia, pneumonia, atelectasis, and surgical site complications requiring revision were included in the analysis. Lung cancer staging was done according to the IASLC 8th edition staging system [9]. Demographic characteristics, complications, and early and long-term pain were evaluated based on the three different surgical techniques applied.
Statistical Analysis
Continuous variables are presented as a mean ± standard deviation, and discrete variables are expressed as frequencies. The demographic and clinical characteristics of the patients were tested for normal distribution using the Kolmogorov-Smirnov test. The t-test was used to calculate the means of these variables in the three groups, and the chi-square test was used to compare morbidity between the three groups. Paired samples were analyzed by using Wilcoxon Test. All analyses were performed using SPSS software (version 22; SPSS Inc., Chicago, IL, USA). P values <0.05 were considered significant and p value <0.016 was considered significant between two groups with Bonferroni correction. Power analysis was 0,86.
Results
Of the 89 patients in the study, 73 (82%) were male, and 16 (18%) were female. The mean age was 54.73 ± 12.14 (range 20-78) years, and the mean length of hospital stay was 6.1 ± 4.03 days. Thirty-six (40.4%) of the patients in the study were operated for benign causes and 53 (59.6%) for malignant disease. There were significant differences in length of hospital stay between the patients operated for benign and malignant disease (p = 0.012). A comparison of the demographic characteristics of the groups is given in table 1. Intercostal closing time differed significantly between the groups (group A: 3.13 ± 0.85 min., group B: 4.48 ± 1.23 min., group C: 5.59 ± 1.10 min; p < 0.001). In benign/malignant diseases subgroup analysis closing time also differed significantly between the groups. P < 0.001).Table 1: Comparison of demographic characteristics of the patients.
No complications occurred during the intercostal muscle flap procedure. However, during the intracostal suture technique, rib fracture occurred in 2 patients while using the hand-held drill. A sternum perforator was used thereafter to avoid the risk of rib fracture. There was no significant between gender and postoperative pain (p = 0,217).
At postoperative day 1, 2, and 3, there were significant differences between the groups in pain scores when at rest and while coughing (p < 0.001). With Bonferroni correction, p < 0,016 were calculated as a significant value to evaluate between two groups. Resting and coughing pain scores were significantly higher in group A compared to groups B and C at postoperative day 1, 2 and 3 (p < 0,001). Group C showed lower resting and coughing pain scores than group B at postoperative day 2 (p = 0.012 and p = 0.008). There was no statistical difference between coughing and resting pain scores of group B and C at day 1 (p = 0.029 and p = 0.057) and day 3 (p = 0.667 and p = 0.700). When benign and malign patient’s pain scores compared separately group A were significantly higher scores. The relationship between surgical procedure and postoperative pain scores are shown in table 2 and figure 3.
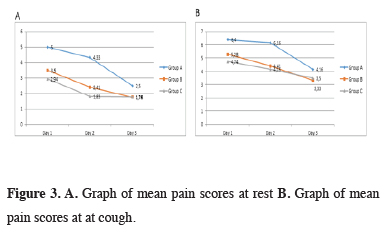 Click Here to Zoom |
Figure 3: A. Graph of mean pain scores at rest B. Graph of mean pain scores at at cough. |
Table 2: Postoperative early period pain comparison between groups.
Totally 26 complications were occurred in 22 patients (24.7%). The complication rate was higher in group A (31.9%) compared to groups B (20%) and C (23.3%), but the difference was nonsignificant (p = 0.603). Atelectasis was more common in group A than in the other groups but it was not significant when compare with the groups (p = 0.027). No statistical difference was found when the complications of patients with the malignant and benign disease were compared based on closure technique (p = 0.690 and p = 0.487) (Table 3). To evaluate secretion retention fiberoptic bronchoscopy was performed in patients with atelectasis.
Table 3: Postoperative complication comparison between groups
Although no statistically significant difference was observed in VAS scores at postoperative 6 months, there was a significant difference in LANSS scores between patients in group A and patients in groups B and C. By Bonferroni correction, Group A found significantly higher rates of patients with LANNS scores > 12 than two other groups (p < 0,001). Between group B and C there was not any siginificant difference (p = 0,317).
That showed LANNS score difference was caused by group A. There were no superiority between intracostal suture technique and intracostal mucles flap technique in LANSS score. A statistically significant difference was found between LANSS scores when the closure techniques were compared among patients with benign and malignant diseases (Table 4). At postoperative six months, 28.1% of the patients (n = 25) required intermittent treatment with nonsteroidal anti-inflammatory drugs. Twenty-nine patients (32.6%) developed post-thoracotomy pain syndrome (PTPS). There was no statistically significant sex difference in terms of prevalence of 6-month pain (22 males [30.1%] vs. 7 females [43.8%]; p = 0.293).
Tablo 4: Comparison of the 6-month pain score between the groups
Discussion
Although there are many studies in the literature about chronic pain, our understanding of the phenomenon remains limited. Despite the development of many new drugs and techniques for relieving pain, it continues to be one of the biggest problems facing medicine today [8,9]. Systemic opioids, nonsteroidal anti-inflammatory drugs, epidural analgesics, and techniques such as intrapleural and intercostal nerve blocks are used to relieve post-thoracotomy pain. However, none of these techniques can adequately control pain in some cases.In the late postoperative period, some patients have complaints of burning, numbness, and stinging, which characterize neurogenic pain. This chronic pain is referred to as PTPS or chronic post-thoracotomy pain (CPTP). PTPS develops at a rate of 30-50% after thoracotomy [10–12]. However, due to the lack of standard criteria for PTPS, the reported incidence ranges considerably. The pain persists for a period of 2 months to 5 years on average. According to the definition by the International Association for the Study of Pain, chronic post-thoracotomy pain persists or recurs for two months or longer after surgery [13], while Cerfolio [14], Kalso [15], and Katz [6] state this period as six months. In our study, we evaluated patients’ pain levels at postoperative six months to assess the effect of different surgical closure techniques on rates of chronic pain, and we determined that 32.6% of the patients have PTPS. Compared to the conventional suture technique, intercostal and intracostal nerve preservation techniques significantly reduced chronic pain.
PTPS is thought to be the result of injury to the intercostal nerves, which transmit pain signals from the chest wall and costal pleura [16]. Pain can arise due to incision shape, muscle separation, intercostal nerve damage, or tube location. The most important of these factors is intercostal nerve damage [2,17,18]. Rib retraction is believed to be a common cause of this damage.
Age, sex, previous surgical interventions, genetic predisposition, and psychosocial factors are thought to influence chronic pain [19]. To date, however, no studies have shown that age and sex are risk factors for chronic post-thoracotomy pain [20]. Similarly, we also detected no correlation between demographic characteristics and pain.
In 2003, Cerfolio [3] showed in a study of 280 patients that the intracostal suture technique significantly reduced pain in the first three months postoperatively compared to the pericostal suture technique (p < 0.001). In a 2005 study, he evaluated ICM cut with ICM flap for preventing nerve damage due to retraction. The patients with ICM flap had significantly less pain at 12 weeks (p = 0.002) [21]. For this reason, we used ICM flaps instead of ICM cut in our study. Bayram et al. [15] compared two intracostal suture techniques, one of which avoided compressing the intercostal nerve, and showed that patients without intercostal nerve compression had less pain at rest and during coughing. In our study, we evaluated the long-term results of a pericostal sutures and intracostal sutures with and without ICM flap. In terms of early postoperative pain, we achieved better results in patients without intercostal nerve compression compared to the conventional method (p < 0.001). Although there was no difference between the intracostal suture techniques with and without nerve compression in terms of pain at postoperative six months, both techniques were superior to the conventional pericostal suture method.
Cerfolio [3] reported that pain is the most important independent factor increasing postoperative morbidity and mortality. In a prospective study by Wu et al. [15] comparing intracostal suture and ICM flap techniques, no difference was observed in terms of early complications (arrhythmia and atelectasis). The authors also reported no difference in postoperative pain scores at rest and while coughing. However, oxycodone use was significantly lower in the ICM flap group during postoperative days 4–7. They detected no significant difference in general complications based on suture technique.
In our study, we noted a significant difference in operative time between the suture technique groups. This was due to the use of a hand-held drill when initially practicing the intracostal suture technique. However, because it extended the procedure time, a sternum perforator was used in subsequent cases. This technique enables holes to be drilled in the ribs rapidly. In addition, using a sternum perforator eliminates the risk of rib fracture and a major injury to the lung parenchyma and heart which can occur when using a drill. Although the intracostal suture procedures result in longer operative times, we do not consider the difference great enough to cause an increased anesthesia risk. Therefore, we believe these techniques are feasible and beneficial for reducing pain in the long term.
Study Limitations
Despite being a prospective study, evaluating patients with malignant and benign pathologies together resulted in heterogeneity in the study sample. There was also heterogeneity regarding disease stage within the malignant disease group. Furthermore, the patients’ postoperative oncological treatments were not considered when evaluating the patients’ pain responses. We have not evaluated analgesic strategies which were used after surgery. Another limitation of the study is the wide age distribution of the patients.
As a conclusion, the intracostal suture technique with ICM flap resulted in less early postoperative pain and chronic pain compared to the conventional technique. Reduced pain, especially in patients with limited respiratory reserve, prevents atelectasis. Our findings indicate that chronic pain can be reduced by preventing intercostal nerve damage. Intracostal suture technique and ICM flap technique have advantages over conventional techniques. However, there are no long-term clinical differences between each other. For this reason, we recommend that these methods be preferred to routine procedures instead of conventional techniques.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Reference
1) El-Hag-Aly MA, Nashy MR. Double edge closure: A novel technique for reducing post-thoracotomy pain. A randomized control study. Interact Cardiovasc Thorac Surg 2015; 21: 630–5.
2) Cerfolio RJ, Price TN, Bryant AS, Bass CS, Bartolucci AA. Intracostal Sutures Decrease the Pain of Thoracotomy. Ann Thorac Surg 2003; 76: 407-11.
3) Cerfolio RJ, Bryant AS, Bass CS, Bartolucci AA. A prospective, double-blinded, randomized trial evaluating the use of preemptive analgesia of the skin before thoracotomy. Ann Thorac Surg 2003; 76: 1055–8.
4) Wang H tang, Liu W, Luo A lun, Ma C, Huang Dr. Y guang. Prevalence and risk factors of chronic post-thoracotomy pain in Chinese patients from peking union medical college hospital. Chin Med J (Engl) 2012; 125: 3033–8.
5) Hughes R, Gao F. Pain control for thoracotomy. Contin Educ Anaesthesia, Crit Care Pain 2005; 5: 56–60.
6) Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain 1996; 12: 50–5.
7) Koc R, Erdemoglu AK. Validity and reliability of the Turkish Self-administered Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) questionnaire. Pain Med 2010; 11: 1107–14.
8) Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain 2005; 6: 149–58.
9) Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 39–51.
10) Bong CL, Samuel M, Ng JM, Ip-Yam C. Effects of preemptive epidural analgesia on post-thoracotomy pain. J Cardiothorac Vasc Anesth 2005; 19: 786–93.
11) Benedetti F, Amanzio M, Casadio C, Filosso PL, Molinatti M, Oliaro A, et al. Postoperative pain and superficial abdominal reflexes after posterolateral thoracotomy. Ann Thorac Surg 1997; 64: 207–10.
12) D’Andrilli A, Ibrahim M, Ciccone AM, Venuta F, De Giacomo T, Massullo D, et al. Intrapleural intercostal nerve block associated with mini-thoracotomy improves pain control after major lung resection. Eur J Cardiothoracic Surg 2006; 29: 790–4.
13) Johnson BE, Cortazar P, Chute JP. Second lung cancers in patients successfully treated for lung cancer. Semin Oncol 1997; 24: 492-9.
14) Merskey H. International association for the study of pain (IASP): subcommittee on taxonomy, classification of chronic pain, description of pain terms. Pain 1986; 3: S29-30.
15) Kalso E, Perttunen K, Kaasinen S. Pain after thoracic surgery. Acta Anaesthesiol Scand 1992; 36: 96–100.
16) Tsaousides T, Gordon W. Cognitive rehabilitation following traumatic brain injury: assessment to treatment. Mt Sinai J Med 2009; 76: 173–81.
17) Takamori S, Yoshida S, Hayashi A, Matsuo T, Mitsuoka M, Shirouzu K. Intraoperative intercostal nerve blockade for postthoracotomy pain. Ann Thorac Surg 2002; 74: 338–41.
18) Debreceni G, Molnar Z, Szelig L, Molnar TF. Continuous epidural or intercostal analgesia following thoracotomy: a prospective randomized double‐blind clinical trial. Acta Anaesthesiol Scand 2003; 47: 1091–5.
19) Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1618–25.



