

2Department of Thoracic Surgery, School of Medicine, Ege University, İzmir, Turkey
3Department of Anesthesiology and Reanimation, Private Ege City Hospital, İzmir, Turkey
4Department of Pathology, School of Medicine, Ege University, İzmir, Turkey DOI : 10.26663/cts.2016.0001
Summary
Background: Cryotherapy is an actual treatment and diagnostic method in many fields of medicine. We aimed to evaluate the acute histopathological effects of cryotherapy application on lung tissue.Materials and Methods: In this study 10 male rabbits were used. After providing single lung ventilation of the rabbits, clamshell incision was performed for exploration. Four different cryotherapy applications were performed to determine the different histopathologic changes on atelectatic and ventilated lung, also the difference between one versus two-cycle applications. After all applications, firstly, air leak was checked, then specimens were taken to analyze the acute histopathologic effects.
Results: After cryotherapy application, no air leak was detected on ventilated rabbit lung with a pressure of 30 cmH2O. There was no bleeding from lung tissue, but macroscopic parenchymal hematoma developed. The depth of necrosis in lung tissue was evaluated with histopathological analysis. In all samples, alveolar edema and congestion were observed but there was no statistical difference between the depth of necrosis regarding with different cryotherapy applications.
Conclusions: This study indicated that different applications of cryotherapy on lung parenchyma is a safe method and do not affect the depth of necrosis in a statistically significant degree.
Introduction
Cryotherapy is a method which has a usage for many organs and diseases especially for malignancies. For medically or surgically inoperable patients with lung cancer, cryotherapy is used sporadically. Recently, transbronchial cryobiopsies are getting more common in the diagnosis of interstitial lung diseases.In this study, we aimed to evaluate air leak and acute histopathological effects on lung caused by the therapy, to guide how to use cryotherapy and adapt this method to a surgical approach.
Methods
Ten male rabbits weighing 944-1386 grams were used and the study was approved by the "Local Ethics Committee of Animal Experiments" of our hospital.For the application, a carbon dioxide cryoprobe which provides a temperature around -40 C° at the probe tip was used. As the probe used for application on human was large for rabbit lung, a probe with a 3 mm tip diameter was obtained (Figure 1).
 Click Here to Zoom |
Figure 1: The probe with a 3 mm tip diameter for the application on rabbit lung. |
Experimental Study Method
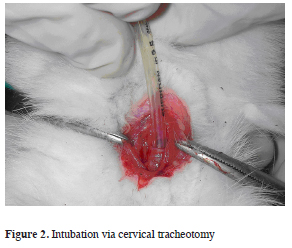
The induction of anesthesia was provided with ketamine (10 mg/kg) and xylazine (3 mg/kg) via 24 gauge cannula which was placed to lateral ear vein of the rabbit. After removal of the hair from the surgical site with cervical incision trachea was dissected, a transverse tracheotomy was done and intubation was provided with a 3 or 3.5 mm diameter endotracheal tube (Figure 2).
 Click Here to Zoom |
Figure 2: Intubation via cervical tracheotomy |
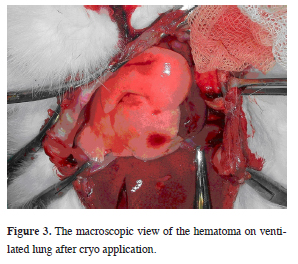
Animals were connected to mechanical ventilator (Servo 900 C, Siemens, Sweden). Following intubation, cisatracurium was used (0.5 mg/kg) intravenously for muscle paralysis. The continuity of anesthesia was secured with ketamine and xylazine bolus infusions. The animals were ventilated with a beginning 10 cmH2O airway pressure in pressure control mode. In order to provide exposure to both hemithorax, a clamshell incision was used, and by pushing the intubation tube forward to right main bronchus single lung ventilation was achieved. First application was performed on the atelectatic lung"s one lobe for a cycle (every cycle contains a freezing period of 20 seconds and a slow thawing period after) and two cycles to another lobe superficially (Figure 3).
 Click Here to Zoom |
Figure 3: The macroscopic view of the hematoma on ventilated lung after cryo application. |
After the thawing finished completely, thoracic cavity was filled with saline solution in order to check air leak and both lungs were ventilated with an increasing airway pressure 5 cmH20 gradually up to 30 cmH20 in every 5 minutes. The same procedure was performed on ventilated right lung, to different lobes, as one and two cycles respectively. To summarize, one cycle cryotherapy was performed for one lobe of the atelectatic left lung (method 1), and two cycles for other lobe (method 2). On the other hand, for ventilated right lung one and two cycle therapies were performed for different lobes (method 3 and method 4 respectively). After air leak check out, lobes were resected for histopathological examination. Animals were sacrificed with high dose intravenous potassium-chloride. The resected lobes were fixed in 10% formalin solution..
Histopathological research
Four-micron sections straight to the cryotherapy application sites were obtained and they were stained with hematoxylin eosin. All the specimens were evaluated by a blinded histopathological examination. The parenchymal injury was evaluated with ocular micrometer measuring the depth of coagulation necrosis.
Statistical method
Because a few number of animals were used for the study, "two-factor analysis of variance" was used. Statistical analysis was performed using SPSS 10.0 (SPSS Inc., Chicago, IL, USA) for windows and p values less than 0.05 were considered significant.
Results
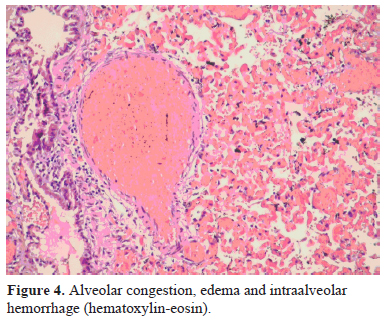
In all specimens", after histopathological evaluation, coagulation necrosis with various levels, alveolar congestion, edema and intraalveolar hemorrhage were observed (Figure 4).
 Click Here to Zoom |
Figure 4: Alveolar congestion, edema and intraalveolar hemorrhage (hematoxylin-eosin). |
The level of coagulation necrosis were evaluated on tissue samples with ocular micrometers (Table 1).
Table 1: The mean value of necrosis on the lung after cryotherapy applications
Air leak was not detected in any of the methods tough airway pressure was increased up to 30 cmH2O. Hemorrhage, from lung parenchyma to the pleural cavity was not seen macroscopically. Only a limited hematoma was observed in the application site.
Medium values of coagulation necrosis in atelectatic left lung was more than it was in the ventilated right lung. There was not any statistical difference between methods although the lowest value of coagulation necrosis was observed in method 3 ("one-cycle" application on ventilated lung).
Discussion
Application of cryotherapy on lung is still controversial although it has a both experimental and routine clinical application area on many organs such as prostate, kidney and liver [1-5]. Due to the specialty of lung tissue, there are two important points to be kept in mind. One is whether the application will cause air leak or not and the other is the histopathological depth of necrosis on alveolar structure? Permpongkosol et al. demonstrated the different histopathological effects of same cryotherapy protocol on kidney, liver and lung [6].At the study of Çakan et al. comparing non-anatomic pulmonary resection via ligasure and harmonic scalpel, the maximum depth of necrosis was 731.5 micron, whereas at our study there were differences when cryotherapy was applied on parenchymal surface versus other methods and depth of necrosis was ranging between 1581-1931 microns [7].
Unlike other solid organs, there are two different options for pulmonary applications. In our study, with one lung ventilation when cryotherapy applied to both ventilated and non-ventilated lung tissue, the depth of necrosis on alveolar tissue and air leak were analysed separately. In addition, the safety of one or two cycles of cryotherapy applications were researched.
In the study of Izumi et al. comparing one and two cycles of cryotherapy on normal lung tissue in a porcine model, there was a significant air leak and hemorrhage detected additionally at the area of the lesion tripled with two cycles of cryoablation versus one cycle [8].
Wang et al. obtained successful results with computed tomography guided percutaneous cryotherapy, on patients with one or more masses that failed with previous therapies (radiation therapy, chemotherapy and /or surgery) or had nonresectable central lung cancer [9]. They inserted cryoprobes although the lung was ventilated and performed the therapy. By 6 months 86% of the treated areas were stable or smaller than the original tumor [10]. Pneumothorax due to the procedure occurred in 12% of the patients and only a few of them required tube thoracostomy.
Nowadays, in thoracic applications, cryotherapy is mostly used in endobronchial tumors. It is a treatment option for patients with an airway obstruction, alternative to laser, diathermia, photodynamic therapy and stent applications [10-16]. Cryotherapy with minimal complication ratios were reported to be a good tolerated method by Maiwant et al. [17-18]. Moorjani et al. indicated that in none of the cases, bronchial perforation or mortality was seen and highlighted the priority of the method [19].
One another usage of cryotherapy is transbronchial cryobiopsy. Although surgical biopsy is still the gold standard for diagnosis of interstitial lung diseases, transbronchial cryobiopsy is getting more common with satisfactory results [20-23]. Pneumothorax and bleeding are the most common complications [21]. Bleeding could be effectively managed in endoscopy unit as reported by Hagmeyer et al. [22].
When cryotherapy is applied directly to the parenchymal surface of lung, it causes pulmonary edema and hemorrhage. For this reason, Izumi et al. applied cryoablation for the treatment of experimentally created air leaks. Their study demonstrated that another potential application of cryotherapy was the control of air leak from dissected raw lung surfaces during lung resection [24]. In our study, cryotherapy probe was not applied inside the parenchymal tissue but on the lung surface and similarly no air leak was detected but only an intraparenchymal hematoma has occurred.
In this study, cryotherapy which comes to order nowadays with its application for cancer treatment, is demonstrated to be effective causing pulmonary hematoma and vascular thrombosis when used superficially and there was no statistical differences between different methods of applications. In conclusion, we are in the opinion that according to our results further studies are needed to investigate usage of cryotherapy.
Declaration of conflicting interests
The author declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The author received no financial support for the research and/or authorship of this article.
Reference
1) Polascik TJ, Nosnik I, Mayes JM, Mouraviev V. Short-term cancer control after primary cryosurgical ablation for clinically localized prostate cancer using third-generation cryotechnology. Urology 2007; 70: 117-21.
2) Chin JL, Lim D, Abdelhady M. Review of primary and salvage cryoablation for prostate cancer. Cancer Control 2007; 14: 231-7.
3) Nakada SY, Jerde TJ, Warner T, Lee FT. Comparison of cryotherapy and nephrectomy in treating implanted VX-2 carcinoma in rabbit kidneys. Investig Urol 2004; 94: 632-6.
4) Janzen NK, Perry KT, Han KR, Kristo B, Raman S, Said JW, et al. The effects of intentional cryoablation and radiofrequency ablation of renal tissue involving the collecting system in a porcine model. J Urol 2007; 173: 1368-74.
5) Ng KK, Lam CM, Poon RT, Shek TW, To JY, Wo YH, et al. Comparison of systemic responses of radiofrequency ablation cryotherapy and surgical resection in a porcine liver model. Ann Surg Oncol 2004; 11: 650-7.
6) Permpongkosol S, Nicol TL, Link RE, Varkarakis I, Khurana H, Zhai QJ, et al. Differences in ablation size in porcine kidney, liver and lung after cryoablation using the same ablation protocol. Am J Roentgenol 2007; 188: 1028-32.
7) Cakan A, Yoldas B, Samancilar O, Ertugrul V, Turhan K, Cagirici U, et al. Ligasure vessel sealing system versus harmonic scalpel for sutureless nonanatomical pulmonary resections in a rabbit model. Eur Surg Res 2009; 43: 24-8.
8) Izumi Y, Oyama T, Ikeda E, Kawamura M, Kobayashi K. The acute effects of transthoracic cryoablation on normal lung evaluated in a porcine model. Ann Thorac Surg 2005; 79: 318-22.
9) Wang H, Littrup PJ, Duan Y, Zhang Y, Feng H, Nie Z. Thoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 procedures. Radiology 2005; 235: 289-98.
10) Maiwand MO, Asimakopoulos G. Cryosurgery for lung cancer: clinical results and technical aspects. Technol Cancer Res Treat 2004; 3: 143-50.
11) Thurer RJ. Cryotherapy in early lung cancer. Chest 2001; 120: 3-5.
12) Mathur PN, Edell E, Sutedja T, Vergnon JM. Treatment of early stage non-small lung cancer. Chest 2003; 123: 176-80.
13) Simoff MJ. Endobronchial management of advanced lung cancer. Cancer Control 2001; 8: 337-43.
14) Vergnon JM, Schmitt T, Alamartine E, Barthelemy JC, Fournel P, Emonot A. Initial combined cryotherapy and irradiation for unresectable non-small cell lung cancer. Chest 1992; 102: 1436-40.
15) Deygas N, Froudarakis M, Ozenne G, Vergnon JM. Cryotherapy in early superficial bronchogenic carcinoma. Chest 2001; 120: 26-31.
16) Asimakopoulos G, Beeson J, Evans J, Maiwand MO. Cryosurgery for malignant endobronchial tumors: analysis of outcome. Chest 2005; 127: 2007-14.
17) Maiwand MO, Evans JM, Beeson JE. The application of cryosurgery in the treatment of lung cancer. Cryobiology 2004; 48: 55-61.
18) Maiwand MO, Zehr KJ, Dyke CM, Peralta M, Tadjkarimi S, Khagani A, et al. The role of cryotherapy for airway complications after lung and heart-lung transplantation. Eur J Cardiothorac Surg 1997; 12: 549-54.
19) Moorjani N, Beeson JE, Evans JM, Maiwand MO. Cryosurgery for the treatment of benign tracheo-bronchial lesions. Interact Cardiovasc Thorac Surg 2004; 3: 547-50.
20) Babiak A, Hetzel J, Krishna G, Fritz P, Moeller P, Balli T, et al. Transbronchial cryobiopsy: a new tool for lung biopsies. Respiration 2009; 78: 203-8.
21) Ganganah O, Guo SL, Chiniah M, Li YS. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: A systematic review and meta-analysis. Respirology 2016; 21: 834-41.
22) Hagmeyer L, Theegarten D, Wohlschläger J, Treml M, Matthes S, Priegnitz C, et al. The role of transbronchial cryobiopsy and surgical lung biopsy in the diagnostic algorithm of interstitial lung disease. Clin Respir J 2016; 10: 589-95.



