2Chronic Diseases Research Center, NOVA Medical School, NOVA University of Lisbon, Portugal
3Department of Interventional Pulmonology, University Clinic Golnik, Golnik, Slovenia DOI : 10.26663/cts.2016.0010
Summary
In the last decade endobronchial ultrasound with real-time guided needle aspiration (EBUS-NA) has been recognized worldwide as a tool with remarkable impact on routine clinical practice. In the present review article, we focus on specific disease-related features of EBUS-NA, including diagnosis and staging of thoracic malignancies, sarcoidosis, tuberculosis and its role in the evaluation of isolated enlarged mediastinal lymph nodes. We also address some less common conditions and discuss emerging and future developments in EBUS technique.Introduction
The mediastinum is an important anatomic region, which contains many vital structures and represents one of the main areas of interest for the Pulmonologist and the Thoracic Surgeon.Several disease processes can affect different mediastinal structures where prompt and accurate diagnosis has a direct impact on treatment and prognosis. In the last decade, convex probe endobronchial ultrasound (CP-EBUS) with real-time guided needle aspiration (NA) has been accepted worldwide as a valuable tool with tremendous impact on routine clinical practice [1-3] (Figure 1). The equipment can be introduced into the airway or in the esophagus as an alternative mediastinal transport pathway as far as the left suprarenal gland [4-7]. The invasiveness of the procedure is much lower than concurrent techniques and EBUS-NA is feasible on outpatient basis under local anesthetic and moderate sedation [8]. A high diagnostic yield and very low complication rate contribute to establish EBUS-NA as the first-choice diagnostic tool in numerous mediastinal disorders [9].
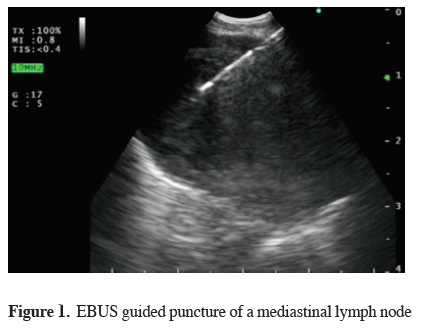 Click Here to Zoom |
Figure 1: EBUS guided puncture of a mediastinal lymph node |
EBUS-NA is most often used in lung cancer staging, for definitive tissue diagnosis in central malignant lesions or for diagnosis of other tumors with its origin or spread into the mediastinum. The obtained specimens can be processed for immunocytochemistry and DNA analysis [10]. Additionally, EBUS-NA provides useful diagnostic samples, including microbiology, for many benign diseases. B-mode and Doppler technique support the selection of the best biopsy spot, or in certain cases, provide complementary data for alternative diagnosis [11-14] (Figure 2).
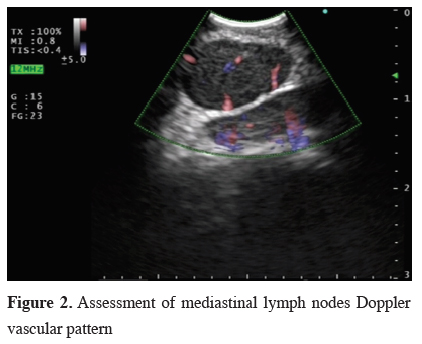 Click Here to Zoom |
Figure 2: Assessment of mediastinal lymph nodes Doppler vascular pattern |
The CP-EBUS instrument and NA technique were already described in detail in countless previous publications [15]. Therefore, in this article, we focus on specific disease-related features of CP-EBUS, including diagnosis and staging of thoracic malignancies, sarcoidosis, tuberculosis and its role in the evaluation of isolated enlarged mediastinal lymph nodes. We also address some less common conditions and discuss emerging developments in CP-EBUS technique.
Lung Cancer Diagnosis
The standard approach to lung cancer diagnosis comprises non-invasive imaging tests followed by invasive procedures such as flexible bronchoscopy or CT-guided transthoracic needle aspiration (CT-TTNA). The selected technique should offer maximum information regarding diagnosis (and staging) with minimum risk to the patient. Flexible bronchoscopy has a high diagnostic yield for endobronchial tumors but for peripheral and extraluminal lesions its sensitivity is rather low [16]. Also, CT-TTNA carries a significant risk of complications especially in central and small lung or mediastinal lesions. In the past, some patients would remain without a definitive diagnosis despite extensive work-up and had to undergo surgical biopsy, not always suitable for those with advanced disease or significant comorbidities.
In the last few years, real-time EBUS-NA proved to be an important option to overcome this situation, and diagnose lung cancer in patients with centrally located tumors. Tournoy et al. [17] reported a sensitivity of 84% and Nakajima et al. [18] obtained 94.1% sensitivity and 94.3% diagnostic accuracy for EBUS-NA in the diagnosis of central lung lesions not visible during routine bronchoscopy. EUS-NA has also been used to diagnose
lung tumors abutting the esophagus [19].The choice between EBUS and EUS depends on the availability of equipment, expertise and the location of the suspicious lesion. In an observational study, combined CP-EBUS with CP-EUS-B (transesophageal endobronchial ultrasound) after failure of conventional techniques, provided a definitive diagnosis in 106 of 121 cases (87.6%) [20]. Dincer et al. [21] even demonstrated that it is feasible and safe to perform EBUS-NA or EUS-NA in ≥10mm lesions not adjacent to the tracheobronchial tree or the esophagus. Of mention, this should be tried only by highly trained operators since there is the potential for reduced yield and higher complications compared to the puncture of abutting lesions.
With endosonography there is also the potential of providing diagnosis and mediastinal staging in the same procedure. It is mandatory that the central tumor is sampled after all lymph nodes are punctured, to avoid needle contamination. In other cases, if the primary lesion cannot be easily assessed, tissue may be acquired from highly suspicious metastatic lymph nodes to diagnose lung cancer.
In 2015, a randomized controlled trial by Navani et al. [22] have shown that EBUS-NA as the initial investigation in diagnosis of suspected lung cancer lesions reduces the time to treatment decision, compared with flexible bronchoscopy or CT-TTNA.
Of mention, linear EBUS does not replace conventional techniques since its size, angulation and image quality do not allow a correct and complete inspection of the airways. Most physicians still use EBUS and the flexible bronchoscope as complementary tools in the same diagnostic procedure.
Lung Cancer Staging
The most common indication for EBUS-NA is lung cancer staging. Identification of metastatic lymph nodes is critical and influences treatment and prognosis. Imaging methods are not sufficiently sensitive to detect lymph node metastasis and in most cases a minimally invasive staging has to be performed. Recently, Ong et al. [23] have shown in N0 PET-CT patients a high false-negative rate detected by EBUS-NA. In a retrospective cohort of 15,316 patients with lung cancer, Ost et al. [24] concluded that when lymph node staging does not meet the international standard guidelines with mediastinal sampling first, patients undergo more diagnostic tests (some unnecessary) with greater morbidity.
Most guidelines recommend endosonography as the initial sampling method for mediastinal lymph node staging [1,25-27]. This is due to the fact that several systematic reviews and meta-analysis showed that EBUS-TBNA has a high pooled sensitivity (88-93%) [26,28-30]. Diagnostic accuracy is at least equivalent to cervical mediastinoscopy in the evaluation of mediastinal lymph node metastasis in lung cancer [31-32]. In a controlled trial, Yasufuku el al. [32] proved that there were no differences between CP-EBUS and mediastinoscopy regarding N stage (EBUS sensitivity, diagnostic yield and negative predictive value were 81%, 93% and 91% and mediastinoscopy 79%, 93% and 90% respectively).
Of notice, some EBUS-NA studies showed limitations regarding its negative predictive value (NPV). This means that a negative result should be further confirmed by other invasive methods especially if the pre-test probability is high [29,30]. Dooms et al. [33] showed that endosonography has an inadequate sensitivity to detect N1 disease in lung cancer (sensitivity of 38% was increased to 73% by adding mediastinoscopy). The risk of false-negative cases is higher if there is: a central tumor, primary tumor is ≥3cm, suspected N2 disease by PET-CT, proven N1 disease or tumor restaging following chemotherapy [1]. Staging by CP-EBUS plus EUS followed by surgical staging (in case of N0) compared to surgical staging alone resulted in higher sensitivity and avoided unnecessary thoracotomies [34,35].
Only in selected cases mediastinoscopy may be omitted and these negative cases should be subjected to follow-up [36].
Another important point regarding lung cancer staging is that all lymph node stations should be systematically investigated because targeted biopsy (guided by chest CT and/or PET scan) may downstage the patient [2]. Further studies are ongoing to test this hypothesis. Recent guidelines advise that at least three different mediastinal nodal stations (4R, 4L, 7) should be sampled in NSCLC patients with an abnormal mediastinum by CT or PET-CT [1].
Since EBUS-NA cannot assess all mediastinal stations it is recommended to be combined with EUS-NA for complete nodal staging [1]. One of the possible advantages of the EBUS scope is that it is officially approved in Europe to use in the upper digestive track. So, in a single procedure, with the same equipment, the operator can perform CP-EBUS and EUS-B [5,6,37] accessing most mediastinal and hilar lymph node stations (except station 5 and 6), improving diagnostic yield, decreasing the negative predictive value and reducing costs [1,38]. The drawback is that EBUS plus EUS-B may increase procedure time, need prolonged sedation protocols, add complexity to the exam and require dedicated skills and training.
The available scientific data suggest that CP-EBUS should be undertaken first, followed by EUS-B [39] because adding EBUS to EUS increases accuracy (from 86.5% to 97.3%) and the opposite did not increase yield or sensitivity.
Since a single dedicated needle is used, N3 stations should be punctured first, followed by N2 and finally N1, to avoid upstaging. Each lymph node should be punctured at least 3 times [
An additional indication for CP-EBUS/EUS may be the detection and puncture of lung cancer metastases in the left adrenal gland. In a retrospective study by Crombag et al. [7] the CP-EBUS scope allowed the identification and transgastric puncture of this anatomic structure in the majority of lung cancer patients with signs of malignant involvement. Prospective data is needed to access feasibility and safety.
Lung Cancer Restaging
Diagnosis of Extrathoracic Cancer
Lymphoma
Rapid on-site evaluation of specimen is helpful to allocate more material to ancillary studies [48,51]. In suspicious cases, further sampling is needed to acquire enough material for immunophenotyping by flow cytometry and fluorescence in situ hybridization (FISH), which provide the basis for non-Hodgkin lymphoma (NHL) subclassification.
EBUS-NA provided diagnosis in 100% of relapsed lymphoma cases and an accurate alternative diagnosis in 97% of patients from the same group [52]. Sensitivity for subtyping to high-grade NHL, low-grade NHL and Hodgkin lymphoma in relapsed and de novo diagnosed patients was 90%, 100% and 79% respectively [52]. Grosu et al. were able to establish the diagnosis and subtype the lymphoma in 67% of new diagnosed patients and in 81% of relapsed lymphoma patients by EBUS-NA using 22G needle [53].
In conclusion, EBUS-NA may be used as an initial procedure for patients with suspected mediastinal lymphoma and may decrease the need for more invasive approaches [48,54,55]. Sensitivity for final diagnosis and subtyping varies, but is more reliable for relapsed than newly diagnosed patients [52,55,56]. Although EBUS-NA can reliably provide alternative diagnosis, negative results do not completely exclude lymphoma [48,52,57,58].
Sarcoidosis
A randomized controlled trial published by Tremblay et al. [59] showed that EBUS-NA in patients with sarcoidosis is able to improve the diagnostic sensitivity in 22% compared to conventional TBNA. Another study [60], proved the enhanced diagnostic yield of EBUS/EUS (80%) versus bronchoscopy (53%) in sarcoidosis, although this trial did not include conventional TBNA. In 2014, Gupta et al. [61] demonstrated that individually EBUS-NA has the highest diagnostic yield (74.5%) and it is even better when combined with TBLB (90.9%) but the diagnostic yield of non-guided TBNA plus endobronchial biopsies and TBLB allows comparable results (85.5%, P > 0.05). In conclusion, EBUS/EUS may be the procedure of choice to diagnose sarcoidosis stage I and II, however those who do not have this equipment can still get a high diagnostic yield by combining the conventional techniques.
Tuberculosis
Isolated Enlarged Lymph Nodes
Although mediastinoscopy was a standard diagnostic procedure, recent the REMEDY clinical trial demonstrated, that EBUS-NA may be recommended as a first line investigation for IMHL [75]. EBUS-NA had 92% sensitivity and 40% negative predictive value for a treatable condition and spared mediastinoscopy in 87% of patients [75]. Moreover, the study also proved cost-effectiveness of such approach. EBUS-NA was also more accurate and cost-effective in comparison to classical TBNA [76]. However, the REMEDY trial, although prospective, found only 5% of patients with reactive lymphadenopathy, which might reflect some kind of pre-selection of patients referred to diagnostic work up [75].
Another single-center study found a much higher prevalence (48%) of reactive lymph nodes [77]. The presence of symptoms was not a reliable predictive factor for differentiation between reactive and pathological IMHL. EBUS-NA had an overall diagnostic accuracy and NPV of 91% and 84.2% respectively [77]. Therefore, the authors recommend surveillance, rather than further invasive procedures in the low risk group of older patients with comorbidities and with maximum lymph node diameter below 20mm, where the NPV may reach 93.8% [77].
Mediastinal Cysts
Future of CP-EBUS
Novel 19G EBUS-NA needles were introduced and may further enhance the diagnostic yield, especially in detection and subtyping of lymphomas (Figure 6).
New EBUS bronchoscopes with higher resolution and smaller size of ultrasonic probe will reach deeper into the bronchial tree and into the upper lobes.
Combined CP-EBUS plus EUS-B procedure will become standard of care for lung cancer mediastinal staging in most interventional units.
Intra-tumoral delivery of cytostatic drugs by transbronchial guided needle injection may improve local control of recurrent central airway and mediastinal cancers, reducing doses and side effects [87-89].
There are also several none-conventional indications where CP-EBUS could occasionally be used, for instance the diagnosis of pulmonary embolism in patients with contraindication for intravenous contrast agent and for detection of non-thrombotic endovascular lesions [90].
Selected studies have addressed the issue of restaging the mediastinum by endosonography. To downstage disease, stage III NSCLC patients may be submitted to neoadjuvant chemoradiotherapy. It is of utmost importance to identify the responders since they are able to benefit from subsequent surgery. Herth et al. [41] published the first EBUS-NA restaging study in lung cancer with an overall sensitivity of 76% and NPV of 20%. Other retrospective studies confirmed the lower sensitivity and NPV of EBUS mediastinal restaging compared to staging [42,43]. In 2015, a prospective trial combined CP-EBUS and EUS with a single echoendoscope for NSCLC restaging had an overall sensitivity, accuracy and NPV of 67%, 81% and 73%, respectively [4]. In view of these data, guidelines suggest that initial restaging may be performed by EBUS-TBNA and/or EUS-(B)-FNA for detection of persistent nodal disease but, if negative, subsequent surgical staging is indicated before radical surgery is attempted (grade C recommendation) [1].
Patients with extrathoracic tumors may develop increased mediastinal or hilar lymph nodes. In most cases, there is the need to acquire material for correct diagnosis and staging. Different scenarios may occur: metastatic dissemination of the extrathoracic cancer, second malignancy, sarcoid-like reaction, reactive lymph nodes or benign disease (e.g. tuberculosis, sarcoidosis). Various authors reported the utility of EBUS-NA for differential diagnosis in patients with a previous extrathoracic malignancy [44-46]. A meta-analysis with 533 patients concluded that EBUS-NA has 85.6% diagnostic accuracy to detect mediastinal lymph node metastases of extrathoracic malignancies and 16% probability to have a negative result (these cases should be confirmed by more invasive methods) [47].
In contrast with high diagnostic yield in lung cancer staging, diagnosis of lymphoma by EBUS-NA is somewhat less reliable [48]. The reason may lie in suboptimal size of biopsy specimens, obtained by dedicated 21G and 22G needles. A small observational study with 22G needles on 25 patients reported sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of 90.9%, 100%, 100% and 92.6%, respectively [49]. In one case, additional mediastinoscopy was performed for further subtyping. Steinfort et al. reported 76% sensitivity with 22G needle, but surgical biopsy to define the subtype was required in 4 patients, which decreases the specificity of EBUS-NA for definitive diagnosis to 57% [50].
Most patients referred for evaluation of suspected pulmonary sarcoidosis present stage I or II disease, with increased lymphadenopaties. Flexible bronchoscopy with transbronchial lung biopsies (TBLB), endobronchial biopsies and non-guided needle aspiration have been the traditional method for diagnosis, when there is an indication for tissue confirmation. These sampling methods may be associated with adverse events such as bleeding or pneumothorax, especially in non-experienced hands. Instead, the detection of non-caseating granulomas can be easily and safely obtained by CP-EBUS or EUS in mediastinal and hilar lymph nodes (Figure 3).
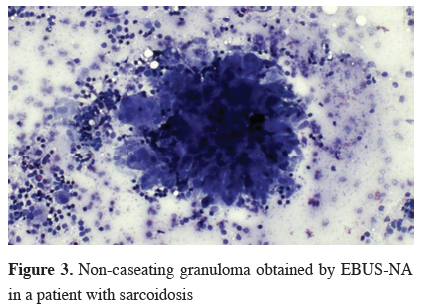
Click Here to ZoomFigure 3: Non-caseating granuloma obtained by EBUS-NA in a patient with sarcoidosis
Intrathoracic tuberculosis lymphadenitis is a frequent companion of pulmonary tuberculosis and may as well represent a form of extrapulmonary tuberculosis on its own. The sputum smear for acid-fast bacilli and microbiological culture are still the mainstay of diagnosis, especially in the era of multi-drug resistant tuberculosis [62]. However, negative sputum, especially in the absence of pulmonary involvement represents a diagnostic challenge. Prompt histological diagnosis and isolation of tuberculosis bacilli was successfully achieved with 19G needles during standard TBNA procedure in HIV positive and negative patients with 83-87% sensitivity and 100% specificity [63,64]. EBUS-NA has an advantage of precise targeting the affected lymph nodes, but at the same time the disadvantage of thinner, 21G and 22G dedicated needles. Recent meta-analyses reported 80% pooled sensitivity and 100% specificity [65,66]. In all studies, 22G needles were used, except in Navani et al. where a combination of 21G and 22G needles had a sensitivity of 94% [67]. The culture and smear positive rates were 54% and 30% respectively [66]. Although the mycobacterium culture has a lower diagnostic yield than cytopathologic investigation, improves overall sensitivity from 72.7% to 95.4% [68]. The same applies to polymerase chain reaction of Mycobacterium tuberculosis from EBUS-NA samples, which increases diagnostic yield in addition to cytopathology and microbiology [69].
Mediastinal or hilar lymphadenopathy is a relatively common finding on chest CT-scans performed for various reasons. The term isolated mediastinal and hilar lymphadenopathy (IMHL) is defined as at least one enlarged lymph node in the mediastinum or hilus without evidence of lung nodule or mass or extrathoracic malignancy. The main diagnostic goal is to recognize a treatable condition like a granulomatous disorder (e.g. tuberculosis, sarcoidosis) or malignancy (e.g. lymphoma, metastasis or rare lymphoproliferative disorders) and to exclude patients with reactive lymph nodes associated with many chronic diseases (e.g. heart failure, bronchiectasis, chronic bronchitis, interstitial lung diseases, etc.) [70-74].
Mediastinal cysts may be classified as bronchogenic, pericardial, or enteric, depending on their lining epithelium. They are often punctured because of diagnostic uncertainty, although they have characteristic anechoic Doppler negative appearance on ultrasound examination [78] (Figure 4). EBUS guided real-time aspiration can be a therapeutic alternative to surgical resection [79,80]. Complications were reported in 16.1% of patients after EBUS-NA of the cyst, mostly as an infection [78,81]. Pericardial cysts are sometimes connected with pericardial sack and an infectious pericarditis might arise, either after intentional or accidental puncture [82,83]. The use of prophylactic antibiotic should be considered before cyst puncture.
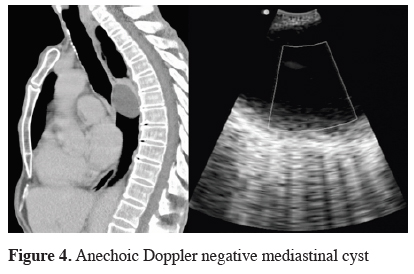
Click Here to ZoomFigure 4: Anechoic Doppler negative mediastinal cyst
CP-EBUS technique and applications are still under further development. New imaging techniques such as elastography may complement the procedure by better selection of the biopsy spot, which can result in more effective and less invasive diagnostic of mediastinal lesions and lung cancer staging [84-86] (Figure 5).
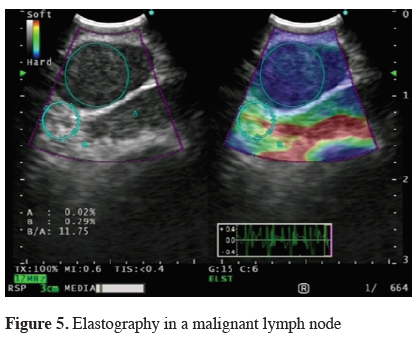
Click Here to ZoomFigure 5: Elastography in a malignant lymph node
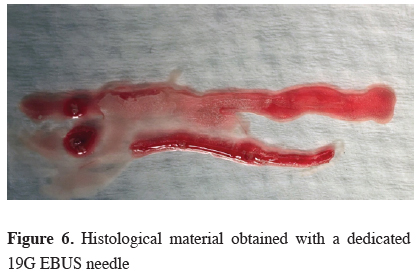
Click Here to ZoomFigure 6: Histological material obtained with a dedicated 19G EBUS needle
Conclusion
As a conclusion, CP-EBUS resulted from the effort of innumerous investigators that believed that ultrasound imaging could be accomplished in the airways.EBUS-NA and EUS-B-NA are now recommended as the first procedures for NSCLC lymph node staging. The technique has also clinical impact for lung cancer diagnosis and restaging of thoracic and extrathoracic malignancies.
In benign diseases, such as tuberculosis and sarcoidosis stage I/II EBUS-NA proved its added value by increasing diagnostic yield.
The international scientific community is still pursuing optimal performance as further studies are performed and new indications are tested. Structured learning programs and supervised training are essential to appropriately disseminate CP-EBUS.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The author received no financial support for the research and/or authorship of this article.
Reference
1) Vilmann P, Clementsen PF, Colella S, Siemsen M, De Leyn P, Dumonceau JM, et al. Combined endobronchial and oesophageal endosonography for the diagnosis and staging of lung cancer. European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Eur Respir J 2015; 46: 40-60.
2) Jenssen C, Annema JT, Clementsen P, Cui XW, Borst MM, Dietrich CF. Ultrasound techniques in the evaluation of the mediastinum, part 2: mediastinal lymph node anatomy and diagnostic reach of ultrasound techniques, clinical work up of neoplastic and inflammatory mediastinal lymphadenopathy using ultrasound techniques and how to learn mediastinal endosonography. J Thorac Dis 2015; 7: E439-58.
3) Slavova-Azmanova NS, Lizama C, Johnson CE, Ludewick HP, Lester L, Karunarathne S, et al. Impact of the introduction of EBUS on time to management decision, complications, and invasive modalities used to diagnose and stage lung cancer: a pragmatic pre-post study. BMC Cancer 2016; 28: 16: 44.
4) Szlubowski A, Zieliński M, Soja J, Filarecka A, Orzechowski S, Pankowski J, et al. Accurate and safe mediastinal restaging by combined endobronchial and endoscopic ultrasound-guided needle aspiration performed by single ultrasound bronchoscope. Eur J Cardiothorac Surg 2014; 46: 262-6.
5) Herth FJ, Krasnik M, Kahn N, Eberhardt R, Ernst A. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest 2010; 138: 790-4.
6) Hwangbo B, Lee GK, Lee HS, Lim KY, Lee SH, Kim HY, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest 2010; 138: 795-802.
7) Crombag LM, Annema JT. Left adrenal gland analysis in lung cancer patients using the endobronchial ultrasound scope: a feasibility trial. Respiration 2016; 91: 235-40.
8) Jeyabalan A, Medford AR. Endobronchial ultrasound-guided transbronchial needle aspiration: patient satisfaction under light conscious sedation. Respiration 2014; 88: 244-50.
9) Bonta PI, Crombag L, Annema JT. Linear endobronchial and endoesophageal ultrasound: a practice change in thoracic medicine. Curr Opin Pulm Med 2016; 22: 281-8.
10) Nakajima T, Anayama T, Koike T, Waddell T, Keshavjee S, Kimura H, et al. Simultaneous isolation of total RNA, DNA, and protein using samples obtained by EBUS-TBNA. J Bronchology Interv Pulmonol 2011; 18: 301-5.
11) Fujiwara T, Yasufuku K, Nakajima T, Chiyo M, Yoshida S, Suzuki M et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest 2010; 138: 641-7.
12) Nakajima T, Anayama T, Shingyoji M, Kimura H, Yoshino I, Yasufuku K. Vascular image patterns of lymph nodes for the prediction of metastatic disease during EBUS-TBNA for mediastinal staging of lung cancer. J Thorac Oncol 2012; 7: 1009-14.
13) Evison M, Morris J, Martin J, Shah R, Barber PV, Booton R, et al. Nodal staging in lung cancer: a risk stratification model for lymph nodes classified as negative by EBUS-TBNA. J Thorac Oncol 2015; 10: 126-33.
14) Memoli JS, El-Bayoumi E, Pastis NJ, Tanner NT, Gomez M, Huggins JT, et al. Using endobronchial ultrasound features to predict lymph node metastasis in patients with lung cancer. Chest 2011; 140: 1550-6.
15) Wahidi MM, Herth F, Yasufuku K, Shepherd RW, Yarmus L, Chawla M, et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016; 149: 816-35.
16) Rivera MP, Mehta AC, Wahidi MM. Establishing the Diagnosis of Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013; 143: 142S-165S.
17) Tournoy KG, Rintoul RC, van Meerbeeck JP, Carroll NR, Praet M, Buttery RC, et al. EBUS-TBNA for the diagnosis of central parenchymal lung lesions not visible at routine bronchoscopy. Lung Cancer 2009; 63:45-9.
18) Nakajima T, Yasufuku K, Fujiwara T, Chiyo M, Sekine Y, Shibuya K, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of intrapulmonary lesions. J Thorac Oncol 2008;3: 985-8.
19) Annema JT, Veseliç M, Rabe KF. EUS-guided FNA of centrally located lung tumours following a non-diagnostic bronchoscopy. Lung Cancer. 2005;48:357-61
20) Bugalho A, Ferreira D, Eberhardt R, Dias SS, Videira PA, Herth FJ, et al. Diagnostic value of endobronchial and endoscopic ultrasound-guided fine needle aspiration for accessible lung cancer lesions after non-diagnostic conventional techniques: a prospective study. BMC Cancer 2013; 13: 130.
21) Dincer HE, Gliksberg EP, Andrade RS. Endoscopic ultrasound and/or endobronchial ultrasound-guided needle biopsy of central intraparenchymal lung lesions not adjacent to airways or esophagus. Endosc Ultrasound 2015; 4: 40-3.
22) Navani N, Nankivell M, Lawrence DR, Lock S4, Makker H5, Baldwin DR, et al. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomized controlled trial. Lancet Respir Med 2015; 3: 282-9.
23) Ong P, Grosu H, Eapen GA, Rodriguez M, Lazarus D, Ost D, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for systematic nodal staging of lung cancer in patients with N0 disease by computed tomography and integrated positron emission tomography-computed tomography. Ann Am Thorac Soc 2015; 12: 415-9.
24) Ost DE, Niu J, S Elting L, Buchholz TA, Giordano SH. Quality gaps and comparative effectiveness in lung cancer staging and diagnosis. Chest 2014; 145: 331-45.
25) Vansteenkiste J, De Ruysscher D, Eberhardt WE, Lim E, Senan S, Felip E, et al. Early and locally advanced nonsmall-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24 (Suppl 6): vi89-vi98.
26) Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, et al. Methods for staging nonsmall cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143 (5 Suppl): e211S-e250S.
27) De Leyn P1, Dooms C, Kuzdzal J, Lardinois D, Passlick B, Rami-Porta R, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for nonsmall-cell lung cancer. Eur J Cardiothorac Surg 2014; 45: 787-98.
28) Gu P, Zhao YZ, Jiang LY, Zhang W, Xin Y, Han BH. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009; 45: 1389-96.
29) Adams K, Shah PL, Edmonds L, Lim E. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009; 64: 757-62.
30) Dong X, Qiu X, Liu Q, Jia J. Endobronchial ultrasound-guided transbronchial needle aspiration in the mediastinal staging of nonsmall cell lung cancer: a meta-analysis. Ann Thorac Surg 2013; 96: 1502-7.
31) Ernst A, Anantham D, Eberhardt R, Krasnik M, Herth FJ. Diagnosis of mediastinal adenopathy - real-time endobronchial ultrasoundguided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008; 3: 577-82.
32) Yasufuku K, Pierre A, Darling G, de Perrot M, Waddell T, Johnston M, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011; 142: 1393-400.
33) Dooms C, Tournoy KG, Schuurbiers O, Decaluwe H, De Ryck F, Verhagen A, et al. Endosonography for mediastinal nodal staging of clinical N1 nonsmall cell lung cancer: a prospective multicenter study. Chest 2015; 147: 209-15.
34) Annema JT, van Meerbeeck JP, Rintoul RC, Dooms C, Deschepper E, Dekkers OM, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010; 304: 2245-52.
35) Rintoul RC, Glover MJ, Jackson C, Hughes V, Tournoy KG, Dooms C, et al. Cost effectiveness of endosonography versus surgical staging in potentially resectable lung cancer: a health economics analysis of the ASTER trial from a European perspective. Thorax 2014; 69: 679-81.
36) Annema JT, De Leyn P, Clementsen P, Siemsen M, Vilmann P. Mediastinoscopy after negative endoscopic mediastinal nodal staging: can it be omitted? Eur Respir J 2015; 46: 1848-9.
37) Oki M, Saka H, Ando M, Tsuboi R, Nakahata M, Oka S, et al. Transbronchial vs transesophageal needle aspiration using an ultrasound bronchoscope for the diagnosis of mediastinal lesions: a randomized study. Chest 2015; 147: 1259-66.
38) Zhang R, Ying K, Shi L, Zhang L, Zhou L. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis. Eur J Cancer 2013; 49: 1860-7.
39) Kang HJ, Hwangbo B, Lee GK, Nam BH, Lee HS, Kim MS, et al. EBUS-centred versus EUS-centred mediastinal staging in lung cancer: a randomised controlled trial. Thorax 2014; 69: 261-8.
40) van der Heijden EH, Casal RF, Trisolini R, Steinfort DP, Hwangbo B, Nakajima T, et al. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014; 88: 500-17.
41) Herth FJ, Annema JT, Eberhardt R, Yasufuku K, Ernst A, Krasnik M, et al. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 2008; 26: 3346-50.
42) Szlubowski A, Herth FJ, Soja J, Kołodziej M, Figura J, Cmiel A, et al. Endobronchial ultrasound-guided needle aspiration in non-small-cell lung cancer restaging verified by the transcervical bilateral extended mediastinal lymphadenectomy: a prospective study. Eur J Cardiothorac Surg 2010; 37: 1180-4.
43) Nasir BS, Bryant AS, Minnich DJ, Wei B, Dransfield MT, Cerfolio RJ. The efficacy of restaging endobronchial ultrasound in patients with non-small cell lung cancer after preoperative therapy. Ann Thorac Surg 2014; 98: 1008-12.
44) Tournoy KG, Govaerts E, Malfait T, Dooms C. Endobronchial ultrasound-guided transbronchial needle biopsy for M1 staging of extrathoracic malignancies. Ann Oncol 2011; 22: 127-31.
45) Navani N, Nankivell M, Woolhouse I, Harrison RN, Munavvar M, Oltmanns U, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of intrathoracic lymphadenopathy in patients with extrathoracic malignancy: a multicenter study. J Thorac Oncol 2011; 6: 1505-9.
46) Parmaksız ET, Caglayan B, Salepci B, Comert SS, Kiral N, Fidan A, et al. The utility of endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal or hilar lymph node evaluation in extrathoracic malignancy: Benign or malignant? Ann Thorac Med 2012; 7: 210-4.
47) Yang B, Li F, Shi W, Liu H, Sun S, Zhang G, Jiao S. Endobronchial ultrasound-guided transbronchial needle biopsy for the diagnosis of intrathoracic lymph node metastases from extrathoracic malignancies: a meta-analysis and systematic review. Respirology 2014; 19: 834-41.
48) Nason KS, Kirchner A, Schuchert MJ, Luketich JD, Christie NA, Pantanowitz L, et al. Endobronchial ultrasound-transbronchial needle aspiration for lymphoma in patients with low suspicion for lung cancer and mediastinal lymphadenopathy. Ann Thorac Surg 2016; 101: 1856-63.
49) Kennedy MP, Jimenez CA, Bruzzi JF, Mhatre AD, Lei X, Giles FJ, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax 2008; 63: 360-5.
50) Steinfort DP, Conron M, Tsui A, Pasricha SR, Renwick WE, Antippa P, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thorac Oncol 2010; 5: 804-9.
51) Ko HM, da Cunha Santos G, Darling G, Pierre A, Yasufuku K, Boerner SL, et al. Diagnosis and subclassification of lymphomas and non-neoplastic lesions involving mediastinal lymph nodes using endobronchial ultrasound-guided transbronchial needle aspiration. Diagn Cytopathol 2013; 41: 1023-30.
52) Moonim MT, Breen R, Fields PA, Santis G. Diagnosis and subtyping of de novo and relapsed mediastinal lymphomas by endobronchial ultrasound needle aspiration. Am J Respir Crit Care Med 2013; 188: 1216-23.
53) Grosu HB, Iliesiu M, Caraway NP, Medeiros LJ, Lei X, Jimenez CA, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis and subtyping of lymphoma. Ann Am Thorac Soc 2015; 12: 1336-44.
54) Korrungruang P, Oki M, Saka H, Kogure Y, Tsuboi R, Oka S, et al. Endobronchial ultrasound-guided transbronchial needle aspiration is useful as an initial procedure for the diagnosis of lymphoma. Respir Investig 2016; 54: 29-34.
55) Marshall CB, Jacob B, Patel S, Sneige N, Jimenez CA, Morice RC, et al. The utility of endobronchial ultrasound-guided transbronchial needle aspiration biopsy in the diagnosis of mediastinal lymphoproliferative disorders. Cancer Cytopathol 2011; 119: 118-26.
56) Iqbal S, DePew ZS, Kurtin PJ, Sykes AM, Johnson GB, Edell ES, et al. Endobronchial ultrasound and lymphoproliferative disorders: a retrospective study. Ann Thorac Surg 2012; 94: 1830-4.
57) Erer OF, Erol S, Anar C, Aydogdu Z, Ozkan SA. Diagnostic yield of EBUS-TBNA for lymphoma and review of the literature. Endosc Ultrasound 2016 Apr 28. doi: 10.4103/2303-9027.180762.
58) Senturk A, Babaoglu E, Kilic H, Hezer H, Dogan HT, Hasanoglu HC, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Asian Pac J Cancer Prev 2014; 15: 4169-73.
59) Tremblay A, Stather DR, MacEachern P, Khalil M, Field SK. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest 2009; 136: 340-46.
60) von Bartheld MB, Dekkers OM, Szlubowski A, Eberhardt R, Herth FJ, in 't Veen JC, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA 2013; 309: 2457-64.
61) Gupta D, Dadhwal DS, Agarwal R, Gupta N, Bal A, Aggarwal AN. Endobronchial Ultrasound Guided TBNA vs. Conventional TBNA in the diagnosis of sarcoidosis. Chest 2014; 146: 547-56.
62) Internal Clinical Guidelines Team (UK). Tuberculosis: Prevention, Diagnosis, Management and Service Organisation. London: National Institute for Health and Care Excellence (UK); 2016 Jan.
63) Bilaçeroğlu S, Günel O, Eriş N, Cağirici U, Mehta AC. Transbronchial needle aspiration in diagnosing intrathoracic tuberculous lymphadenitis. Chest 2004; 126: 259-67.
64) Harkin TJ, Ciotoli C, Addrizzo-Harris DJ, Naidich DP, Jagirdar J, Rom WN. Transbronchial needle aspiration in patients infected with HIV. Am J Respir Crit Care Med 1998; 157: 1913-8.
65) Ye W, Zhang R, Xu X, Liu Y, Ying K. Diagnostic efficacy and safety of endobronchial ultrasound-guided transbronchial needle aspiration in intrathoracic tuberculosis: a meta-analysis. J Ultrasound Med 2015; 34: 1645-50.
66) Li W, Zhang T, Chen Y, Liu C, Peng W. Diagnostic value of convex probe endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal tuberculous lymphadenitis: a systematic review and meta-analysis. Med Sci Monit 2015; 21:2 064-72.
67) Navani N, Molyneaux PL, Breen RA, Connell DW, Jepson A, Nankivell M, et al. Utility of endobronchial ultrasound-guided transbronchial needle aspiration in patients with tuberculous intrathoracic lymphadenopathy: a multicentre study. Thorax 2011; 66: 889-93.
68) Kiral N, Caglayan B, Salepci B, Torun Parmaksiz E, Fidan A, Comert SS, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in diagnosing intrathoracic tuberculous lymphadenitis. Med Ultrason 2015; 17: 333-8.
69) Eom JS, Mok JH, Lee MK, Lee K, Kim MJ, Jang SM, et al. Efficacy of TB-PCR using EBUS-TBNA samples in patients with intrathoracic granulomatous lymphadenopathy. BMC Pulm Med 2015; 15: 166.
70) Slanetz PJ, Truong M, Shepard JA, Trotman-Dickenson B, Drucker E, McLoud TC. Mediastinal lymphadenopathy and hazy mediastinal fat: new CT findings of congestive heart failure. AJR Am J Roentgenol 1998; 171: 1307-9.
71) Erly WK, Borders RJ, Outwater EK, Zaetta JM, Borders GT. Location, size, and distribution of mediastinal lymph node enlargement in chronic congestive heart failure. J Comput Assist Tomogr 2003; 27: 485-9.
72) Chabbert V, Canevet G, Baixas C, Galinier M, Deken V, Duhamel A, et al. Mediastinal lymphadenopathy in congestive heart failure: a sequential CT evaluation with clinical and echocardiographic correlations. Eur Radiol 2004; 14: 881-9.
73) Kirchner J, Kirchner EM, Goltz JP, Obermann A, Kickuth R. Enlarged hilar and mediastinal lymph nodes in chronic obstructive pulmonary disease. J Med Imaging Radiat Oncol 2010; 54: 333-8.
74) Kirchner J, Kirchner EM, Goltz JP, Lorenz VW, Kickuth R. Prevalence of enlarged mediastinal lymph nodes in heavy smokers - a comparative study. Eur Radiol 2011; 21: 1594-9.
75) Navani N, Lawrence DR, Kolvekar S, Hayward M, McAsey D, Kocjan G, et al. Endobronchial ultrasound-guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. Am J Respir Crit Care Med 2012; 186: 255-60.
76) Grove DA, Bechara RI, Josephs JS, Berkowitz DM. Comparative cost analysis of endobronchial ultrasound-guided and blind TBNA in the evaluation of hilar and mediastinal lymphadenopathy. J Bronchology Interv Pulmonol 2012; 19: 182-7.
77) Evison M, Crosbie PA, Morris J, Martin J, Barber PV, Booton R. A study of patients with isolated mediastinal and hilar lymphadenopathy undergoing EBUS-TBNA. BMJ Open Respir Res 2014; 1: e000040.
78) Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic systs: systematic review of case reports. J Bronchology Interv Pulmonol 2015; 22: 195-203.
79) Twehues A, Islam S. Cystic lesions of the thorax: role of endobronchial ultrasound-guided transbronchial needle aspiration. J Bronchology Interv Pulmonol 2011; 18: 265-8.
80) Kirmani B, Kirmani B, Sogliani F. Should asymptomatic bronchogenic cysts in adults be treated conservatively or with surgery? Interact Cardiovasc Thorac Surg 2010; 11: 649-59.
81) Hong G, Song J, Lee KJ, Jeon K, Koh WJ, Suh GY, et al. Bronchogenic cyst rupture and pneumonia after endobronchial ultrasound-guided transbronchial needle aspiration: a case report. Tuberc Respir Dis (Seoul) 2013; 74: 177-80.
82) Haas AR. Infectious complications from full extension endobronchial ultrasound transbronchial needle aspiration. Eur Respir J 2009; 33: 935-8.
83) Lee HY, Kim J, Jo YS, Park YS. Bacterial pericarditis as a fatal complication after endobronchial ultrasound-guided transbronchial needle aspiration. Eur J Cardiothorac Surg 2015; 48: 630-2.
84) Izumo T, Sasada S, Chavez C, Watanabe J, Katsurada M, Matsumoto Y, et al. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol 2014; 44: 956-62.
85) Nakajima T, Inage T, Sata Y, Morimoto J, Tagawa T, Suzuki H, et al. Elastography for predicting and localizing nodal metastases during endobronchial ultrasound. Respiration 2015; 90: 499-506.
86) Rozman A, Malovrh MM, Adamic K, Subic T, Kovac V, Flezar M, et al. Endobronchial ultrasound elastography strain ratio for mediastinal lymph node diagnosis. Radiol Oncol 2015; 49: 334-40.
87) Khan F, Anker CJ, Garrison G, Kinsey CM. Endobronchial ultrasound-guided transbronchial needle injection for local control of recurrent nonsmall cell lung cancer. Ann Am Thorac Soc 2015; 12: 101-4.
88) Mehta HJ, Begnaud A, Penley AM, Wynne J, Malhotra P, Fernandez-Bussy S, et al. Treatment of isolated mediastinal and hilar recurrence of lung cancer with bronchoscopic endobronchial ultrasound guided intratumoral injection of chemotherapy with cisplatin. Lung Cancer 2015; 90: 542-547.






