Summary
There are limited data on the relapsing polychondritis (RP). RP is an uncommon autoimmune disorder that is characterized by recurrent inflammation and destruction of the cartilage and connective tissue in various parts of the body with a high risk of misdiagnosis. Here we present a rare case of a 43-year-old female with relapsing polychondritis with tracheal involvement.Introduction
Relapsing polychondritis (RP) is a rare, autoimmune, multisystem disease characterized by antibodies to type II collagen in its pathogenesis, which is accompanied by episodic inflammatory attacks in articular and non-articular cartilage tissue [1,2]. Airway manifestations are the most comment cause of morbidity and mortality in the disease [3].Case Presentation
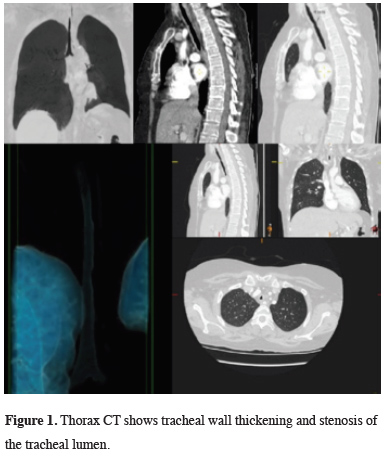
A 43-year-old female patient was admitted to our emergency department in 2015 with nausea, vomiting, and sudden respiratory distress. The patient with severe tachypnea and stridor revealed carbon dioxide (CO2) retention and respiratory acidosis in the arterial blood gas. The patient, whose airway safety was at risk, was intubated under emergency conditions and admitted to the anesthesia intensive care unit. The patient who did not have a disease associated with lung parenchyma, which would lead to carbon dioxide retention. In the computed tomography of the thorax (CT) tracheal wall thickening and tracheal lumen narrowing were detected (Figure 1).
 Click Here to Zoom |
Figure 1: Thorax CT shows tracheal wall thickening and stenosis of the tracheal lumen. |
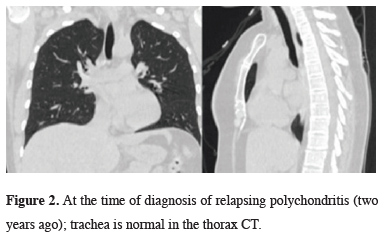
It was understood from the hospital records that the patient was diagnosed with RP due to swelling and edema in her ear 2 years ago. In the thorax tomography taken at this date, there was no thickening and stenosis in the wall of the trachea (Figure 2).
 Click Here to Zoom |
Figure 2: At the time of diagnosis of relapsing polychondritis (two years ago); trachea is normal in the thorax CT. |
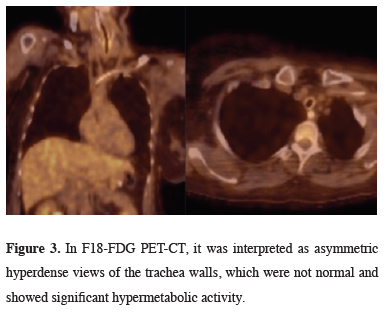
In PET/CT taken after thorax tomography, thick and asymmetric hyperdense views belonging to the tracheal walls, which did not show significant hypermetabolic activity, were detected (Figure 3).
 Click Here to Zoom |
Figure 3: In F18-FDG PET-CT, it was interpreted as asymmetric hyperdense views of the trachea walls, which were not normal and showed significant hypermetabolic activity. |
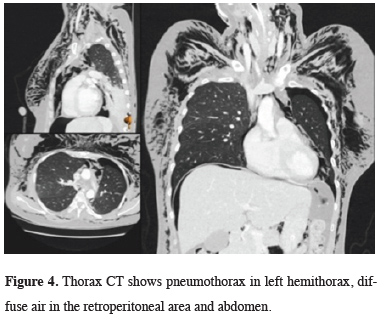
The patient was intervened with a rigid bronchoscope numbered 6.5. It was observed that the airways were hyperemic and edematous. No formation taking up endobronchial lesion was detected. However, during the maneuver with a rigid bronchoscope, the tracheal lumen was seen to collapse again. Tracheal stent implantation was planned for the patient whose extubation attempt was unsuccessful. During the stent application, the patient who developed CO2 retention stent attempt to the patient failed. The patient was intubated and returned to the anesthesia intensive care unit. Upon widespread development of subcutaneous emphysema, control thorax and abdominal CT were taken. There was pneumothorax in the left hemithorax, widespread air was detected in the retroperitoneal region and abdomen (Figure 4).
 Click Here to Zoom |
Figure 4: Thorax CT shows pneumothorax in left hemithorax, diffuse air in the retroperitoneal area and abdomen. |
Tube thoracostomy was performed to the left hemithorax. The patient underwent laparoscopy and the air in the abdomen was drained, but no perforation was detected. After re-bronchoscopy, mucosal laceration was detected in the left main bronchus in an area of approximately 0.5 cm. The chest drain was removed on the 6th day after the procedure after the follow-up of the patient, due to the expansion of the chest x-ray and no air and fluid drainage from the chest tube. The patient, whose medical treatment is continued in the intensive care unit; On the 11th day after laparoscopy, pseudomonas aeriginosa growth in bronchoalveolar lavage (BAL) culture. The patient died on the 28th day due to uncontrolled infection due to sepsis.
Written informed consent was obtained from the patient for publication of her data.
Discussion
RP is a systemic disease causing inflammation of cartilaginous structure in multiple organs [4]. RP progresses as attacks. Our patient had three known attacks. Our patient had her first RP attack in 2013 with edema and swelling on the auricle. Edema develops in the airway during the active period of the disease, and fibrous tissue develops due to chronic inflammation in the late period and the airway collapses due to the destruction of the laryngotracheal cartilages [5]. The estimated incidence is 3.5/1,000,000/year [6]. Airway involvement is present in up to 50% of patients with RP and is a major cause of morbidity and mortality [7]. PET/CT is a potentially powerful tool for the early diagnosis of RP, especially in patients without easily biopsied organ involvement [8]. This method also facilitates the evaluation of disease coverage and disease activity. PET/CT examinations performed in our patient were interpreted as thick and symmetrical hyperdense views belonging to the tracheal walls, which did not show significant hypermetabolic activity. Lung infections develop more frequently secondary to obstruction, which occurs as a result of infection and scarring [4]. In our patient who developed respiratory tract infection during follow-up in the intensive care unit, Pseudomonas aeruginosa was grown in the bronchoalveolar lavage taken during bronchoscopy. The patient died on the 28th day of her hospitalization due to sepsis.In conclusion, relapsing polychondritis is a recurring inflammatory disorder of unknown etiology causing inflammatory reactions in the cartilaginous structures of the nose, ears, trachea, and the joints. Larynx, trachea, and bronchi are most frequently affected. Due to the rarity of the disease, there is no standardized treatment approach, and the treatment is guided by the clinical presentation and the severity of the disease [9]. The presented case was a patient who was diagnosed with relapsing polychondritis 2 years ago and developed tracheal involvement while under treatment, resulting in death. Detection of respiratory involvement at an early stage can significantly reduce mortality.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Reference
1) Foidart JM, Abe S, Martin GR, Zizic TM, Barnett EV, Lawley TJ, Katz SI. Antibodies to type II collagen in relapsing polychondritis. N Engl J Med 1978;929:1203-7.
2) Giroux L, Paquin F, Guerard-Desjardins MJ, Lefaivre A. Relapsing polychondritis: an autoimmune disease. Semin Arthritis Rheum 1983;13:182-7.
3) Goard C, Kadri S. Critical airway involvement in replasing polychondritis. BMJ Case Rep 2014. doi:10.1136/bcr-2014-205036.
4) Spraggs P, Tostevin P, Howard D. Management of laryngotracheobronchial sequelae and complications of relapsing polychondritis. Laryngoscope 1997;107:936-41.
5) Ferrada MA, Grayson PC, Banerjee S, Sikora KA, Colbert RA, Sinaii N et al. Patient perception of disease-related systems and complications in relapsing polychondrits. Arthritis Care Res 2018;70:1124-31.
6) Peebo, B.B.; Peebo, M.; Frennesson, C. Relapsing polychondritis: a rare disease with varying symptoms. Acta Ophthalmol Scand 2004;82:472-5.
7) Tretham DE, Le CH. Relapsing polychondritis: clinical re¬view. Ann Intern Med 1998;192:114- 22.






