

Summary
Background: Lung cancer is a leading cause of cancer-related deaths worldwide, and the majority of lung cancer deaths are due to non-small cell cancers. The most important indicator of prognosis in lung cancer is the tumor stage. In recent years, studies investigating the correlation between cancer development, prognosis, and inflammation have increased. In our study, the prognostic efficacy of the systemic immune-inflammation index (SSI) was investigated in patients with surgically treated nonsmall cell lung cancer in pathologic stage III-A, according to the eighth edition TNM stage classification.Materials and Methods: Following the approval of the local ethics committee, the data of patients with stage III-A lung cancer who were operated on in our clinic between January 2010 and December 2019 were retrospectively analyzed. In accordance with the definitions in the literature, systemic immune-inflammation index groups were calculated using the following formula: (neutrophil count) x (platelet count) / (lymphocyte count) in the preoperative complete blood count tests of patients; the cut-off value was determined by ROC analysis. According to this value, high and low SSI groups were created. The survival difference between the groups was determined by Kaplan–Meier, log-rank, and Cox regression analysis.
Results: A total of 181 patients were included in the study -27 women and 154 men. The median age was 62 (age range: 25 - 82), and the median tumor diameter was 4.5 cm (0.1 - 10 cm). The cut-off value for the SII was calculated as 1,046.105 by ROC analysis. There were 27 patients in the high SSI group and 154 patients in low SSI group. Survival was significantly worse in the high SSI group (P = 0.02, HR: 1.9, 95% CI). In addition, survival was significantly worse in the pneumonectomy group (P = 0.01).
Conclusions: Using the SSI is a simple and inexpensive way to predict prognosis in patients with stage III-A lung cancer who are treated surgically.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide [1]. Although the most important prognostic factor of lung cancer is tumor stage, attempts have been made to determine additional prognostic markers. In the literature, some studies investigating the relationship between inflammation, cancer development, and prognosis of malignancy in various cancer types have been published [2-4]. The systemic immune-inflammation index (SII) is calculated with values obtained from a complete blood count (CBC) test, and it used in prognosis prediction in various cancers. The SII calculation formula is as follows: (platelet count) x (neutrophil count) / (lymphocyte count) [5].The aim of our study is to determine whether there is a correlation between SII and prognosis in surgically treated stage III-A (according to the eighth edition TNM stage classification) non-small cell lung cancers (NSCLC).
Methods
Patient selectionAccording to approval of the local ethic committee (2020-37), the data of the patients who were operated with the diagnosis of non-small cell lung cancer (NSCLC) in our clinic between January 2010 and December 2019 were retrospectively analyzed. Those with stage III-A (T1N2M0, T2N2M0, T3N1M0, T4N1M0, T4N0M0) according to the eighth TNM staging of pathological staging were included the study. The following patients were not included the study; patients who received neo-adjuvant therapy, who had active infection, who could not receive adjuvant therapy and patients whose follow-up records could not be obtained. The SII values of patients were calculated as (platelet count) x (neutrophil count) / (lymphocyte count) in accordance with previous study in the literature. For this, preoperative CBC tests were used. The cut-off value for SII was calculated by Receiver Operator Characteristics (ROC) analysis and high and low SII groups were created based on this value. The data of patients were analyzed according to age, gender, lymph node metastasis, surgery performed and SII cut-off values.
Statistical Analysis
All analyses were performed with the SPPS program, version 20 (IBM, USA). Overall survival (OS) was defined as the date from surgery to the date of death or, for living patients, the date of study. Overall survival analysis was performed using the Kaplan–Meier method, and the significance of the difference in survival between groups was investigated using the log-rank and Cox regression test methods. All analyses were performed in 95% confidence intervals (CI), two-sided P values were calculated, and P < 0.05 value was considered statistically significant.
Results
Descriptive analysesA total of 181 patients who fulfilled the inclusion criteria were included in the study. The demographic and clinical-pathologic features of patients are provided in Table 1.
Table 1: The clinico-pathologic/demographic features and p values of patients, n=181.
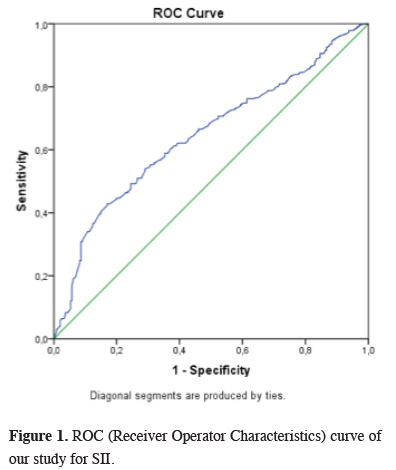
Twenty-seven patients (14.9%) were female, and 154 patients (85.1%) were male. The median age was 62 (age range: 35 - 82), and the median tumor diameter was 4.5 cm (0.9 - 10 cm). In histopathological examinations, N2 station lymph node metastasis was detected in 94 patients (51.9%), and N1 was detected in 41 patients (22.7%). The cut-off value of SII in our series was calculated as 1,046 x 105 by ROC analysis (Figure 1). When groups were formed according to this value, 27 patients were in the high SII group and 154 patients in the low SII group. The largest subgroup of stage III-A of our series according to the eighth edition TNM stage classification was T1N2M0, which included 61 patients (33.7%). Visceral pleural invasion was detected in 77 patients (42.6%) and parietal pleural invasion in nine patients (4.9%).
 Click Here to Zoom |
Figure 1: ROC (Receiver Operator Characteristics) curve of our study for SII. |
Survival analysis
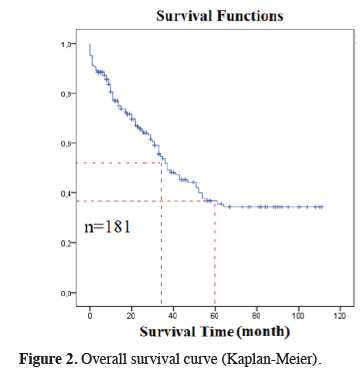
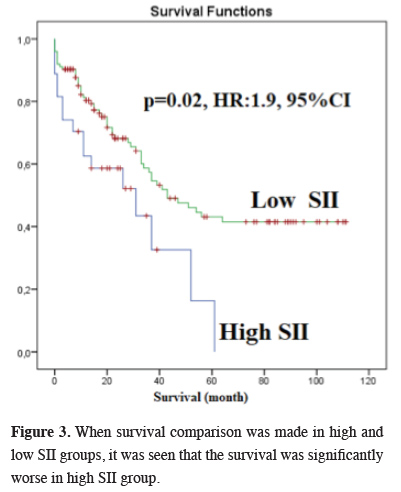
The median survival in our study was 37 months (26.4 - 47.5), and the five-year OS was 35.5% (Figure 2). There was no correlation between gender and survival. The median survival was 31 months in the high SII group and 43 months in the low SII group, and the difference was statistically significant (P = 0.02, HR:1.9, 1.1-3.4, 95% CI) (Figure 3). There was no statistically significant difference between survival and pleural invasion status and age. Survival was significantly worse in the pneumonectomy group and in the T2N2M0 subgroup (P = 0.001 and 0.04, respectively).
 Click Here to Zoom |
Figure 2: Overall survival curve (Kaplan-Meier). |
 Click Here to Zoom |
Figure 3: When survival comparison was made in high and low SII groups, it was seen that the survival was significantly worse in high SII group. |
Discussion
Lung cancer is one of the leading causes of cancer-related deaths worldwide; nearly 80% of lung cancer deaths are due to NSCLC [6]. Recently, serum inflammatory parameters have been emphasized in studies as a way to determine the prognosis of variable cancers. In the literature, studies have indicated a correlation between high platelet-lymphocyte ratio (PLR) and high neutrophil-lymphocyte ratio (NLR) and poor prognosis. Some studies have reported that neutrophils were increased by the cytokine effect in malignant tumors and play an important role in tumorigenesis and angiogenesis [7-9]. Other studies have shown that tumor cells were protected from the phagocytic effect of macrophages by complexing with the platelet; therefore, the serum platelet count indicated tumor aggressiveness [10,11]. Within regard to the number of lymphocytes, the situation reverses because lymphocytes have an antitumor effect. Some studies have shown that a high lymphocyte count is associated with good survival in patients with a malignant tumor [12,13].The SII is a combination of these parameters and is calculated by platelet, neutrophil, and lymphocyte counts in preoperative CBC tests of patients. Gao et al reported that the SII was a strong indicator of prognosis in their studies investigating the correlation between operable non-small cell lung cancers and the SII [14]. Hong et al. showed a significant correlation between the SII, serum LDH level, tumor stage, and survival in small cell lung cancers [15]. Guo et al found that the SII was a superior indicator in terms of prognosis compared to NLR and PLR in surgically resected lung cancer, especially in the adenocarcinoma subgroup [16]. Another study investigating survival in advanced stage lung cancers reported that disease- free survival (DFS) and OS were significantly worse in the high SII group than in the low SII group [5]. Deng et al found worse outcomes in terms of DFS and OS in the high SII group in patients with advanced lung adenocarcinoma treated with tyrosine kinase inhibitors [17]. In a meta-analysis related to the subject, it was concluded that the SII is a poor prognostic factor for lung cancer [18]. In our study, we aimed to provide prognosis homogeneity by selecting cancer patients at the same disease stage. In accordance with the literature, we determined the SII cut-off value for our patient series by ROC analysis, and we formed a high and a low SII group. In the high SII group, median survival and five-year OS were significantly worse. Other significant factors in poor prognosis in our study were pneumonectomy and the T2N2M0 subgroup.
The limitations of our study are that it was single-centered and retrospective and the number of patients included was relatively low.
In conclusion, the SII can be used as a simple, inexpensive, and easily applied marker in the prediction of prognosis of patients with stage III-A lung cancer who are treated surgically. However, this result needs to be supported by prospective, randomized, and multicenter studies.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Reference
1) Knight SB, Crosbie PA, Balata H, Chudziak J, Hussell T, Dive C. Progress and prospects of early detection in lung cancer. Open Biol 2017;7:170070.
2) Deng M, Ma X, Liang X, Zhu C, Wang M. Are pretreatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio useful in predicting the outcomes of patients with small-cell lung cancer? Oncotarget 2017;8:37200-7.
3) Mantovani, A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008;454:436-44.
4) Elyasinia F, Keramati MR, Ahmadi F, Rezaei S, Ashouri M, Parsaei R et al. Neutrophil lymphocyte ratio in different stages of breast cancer. Acta Med Iran 2017;55:228-32.
5) Guo D, Zhang J, Jing W, Liu J, Zhu H, Fu L, et al. Prognostic value of systemic immune inflammation index in patients with advanced non-small-cell lung cancer. Future Oncol 2018;14:2643-50.
6) Aytekin I, Sanli M, Isik AF, Tuncozgur B, Ulusan A, Bakir K et al. Outcomes after lobectomy and pneumonectomy in lung cancer patients aged 70 years or older. Turk J Med Sci 2017;47:307-12.
7) Mantovani, A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008;454:436-44.
8) Goh BK, Kam JH, Lee SY, Chan CY, Allen JC, Jeyaraj P et al. Significance of neutrophil-to lymphocyte ratio, platelet-to-lymphocyte ratio and prognostic nutrition index as preoperative predictors of early mortality after liver resection for huge (>/= 10 cm) hepatocellular carcinoma. J Surg Oncol 2016;113:621-7.
9) Van Egmond M, Bakema JE. Neutrophils as effector cells for antibody-based immunotherapy of cancer. Semin Cancer Biol 2013;23:190-9.
10) Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost 2015;114:449-58.
11) Ding N, Pang Z, Shen H, Ni Y, Du J, Liu Q. The prognostic value of PLR in lung cancer, a meta-analysis based on results from a large consecutive cohort. Sci Rep 2016;6:34823.
12) Chen Y, Wang W, Zhang X, Yu X, Xi K, Wen Y et al. Prognostic significance of combined preoperative platelet-to-lymphocyte ratio and lymphocyte-to-monocyte ratio in patients undergoing surgery with stage IB non-small-cell lung cancer. Cancer Manag Res 2018;10:5411-22.
13) Mao Y, Chen D, Duan S, Zhao Y, Wu C, Zhu F et al. Prognostic impact of pretreatment lymphocyte-to-monocyte ratio in advanced epithelial cancers: a meta-analysis. Cancer Cell Int 2018;18:201.
14) Gao Y, Zhang H, Li Y, Wang D, Ma Y, Chen Q. Preoperative increased systemic immune inflammation index predicts poor prognosis in patients with operable non-small cell lung cancer. Clin Chim Acta 2018;484:272-7.
15) Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic Immune-inflammation Index, based on Platelet Counts and Neutrophil Lymphocyte Ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med 2015;236:297-304.
16) Guo W, Cai S, Zhang F, Shao F, Zhang G, Zhou Y et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected non-small cell lung cancer. Thorac Cancer 2019;10:761-8.



