Summary
A 35-year-old female with a reproductive cough, dyspnea, stridor, and intermittent hemoptysis was evaluated by fiberoptic bronchoscopy (FOB). An endobronchial lesion was observed 2 cm distal to the vocal cords, occluding two-thirds of the tracheal lumen. Rigid bronchoscopy and total excision of the lesion with cryosurgery were planned. Under general anesthesia, a 14-mm rigid bronchoscope (RB) was introduced. As a facilitative new technique, the patient was intubated with an 18F Foley catheter, which was introduced close to the RB. The cuff of the catheter was then inflated distally to the lesion. Subsequently, the catheter was connected to a mechanical ventilator to ensure the safety of the airway while preventing hypoxia. Total excision of the mass was performed via cryosurgery. Immediately after, the catheter balloon was deflated, and a fiberoptic bronchoscope was introduced through the RB to explore the distal bronchial system for aspirated tissue fragments and secretions. With this facilitative approach, the distal airway was kept clean of secretions and tissue fragments. The final pathology of the mass was granulomatous inflammation and necrosis, and the patient was referred to a tuberculosis clinic for medical treatment.Introduction
Interventional bronchoscopy procedures, used by both interventional pulmonologists and thoracic surgeons, have a high success rate, with low morbidity and mortality. Rigid bronchoscopy with cryosurgery and/or argon plasma coagulation is the most common treatment method for endobronchial occlusive lesions [1]. Most of these lesions are liable to bleed, and the bronchial tree distal to the lesion may occlude with blood cloths, which can lead to hypoxia. The development of hypoxia during the procedure complicates this already challenging intervention. There are specific suction catheters and thin lumen intubation tubes designed for use in rigid bronchoscopy procedures [2].In this case report, we used a simple Foley’s catheter for intubation as a facilitative technique to provide distal airway safety while avoiding hypoxia in cryosurgery of the large endotracheal lesion.
Case Presentation
A 35-year-old woman was admitted to our clinic with a reproductive cough, night sweats, weight loss, mild intermittent hemoptysis, weakness, and progressive dyspnea and stridor. The patient was first admitted to hospital 3 months earlier. Since then, she had been misdiagnosed with asthma and received medical treatment. In the patient’s family history, her mother and aunt were both received medical treatment for pulmonary tuberculosis. The patient was admitted to an otolaryngologist, and fiber laryngoscopy performed by him revealed an endotracheal mass 1.5 cm in size distal to the vocal cords. Neck computed tomography revealed a 12 × 10 × 12 mm polypoid soft tissue mass with lobulated margins on the posterolateral wall of the trachea (Figure 1).
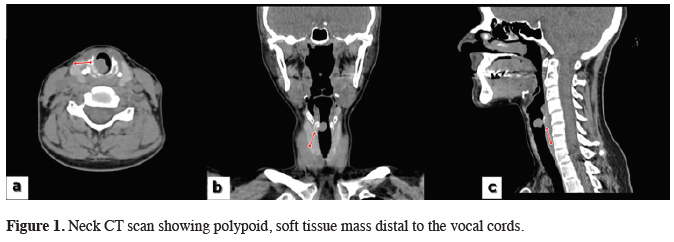 Click Here to Zoom |
Figure 1: Neck CT scan showing polypoid, soft tissue mass distal to the vocal cords. |
Thorax tomography revealed nothing else of note, with no suspicious parenchymal lesions. Three sputum samples obtained on three consecutive days were negative for acid-fast bacillus (AFB) staining. Fiberoptic bronchoscopy was then performed and revealed a mass prone to bleeding 2 cm below the vocal cords. The tracheal lumen hardly allowed the FOB to pass distal to the lesion. There were no other abnormal findings in the tracheobronchial tree. The macroscopic findings suggested a benign tracheal lesion. A biopsy was not performed to avoid bleeding. A bronchoalveolar lavage sample was also negative for AFB staining. Rigid bronchoscopy and cryosurgery were planned.
Under general anesthesia, a 14-mm RB was introduced. A polypoid lesion occluding nearly three-quarters of the tracheal lumen, originating from the right anterolateral tracheal wall was observed 2 cm distal to the vocal cords. We had to urgently secure the airway, as the lesion was bleeding profusely during the exploration with the RB. To secure the distal airway from blood and to maintain ventilation, the patient was intubated with an 18F Foley catheter under guidance of the RB. The catheter was introduced through the vocal cords adjacent to the RB (Figure 2).
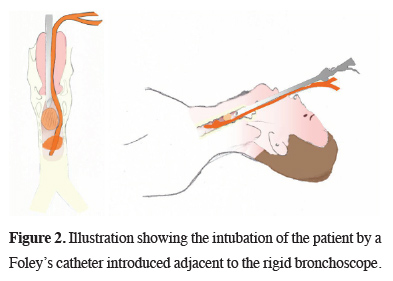 Click Here to Zoom |
Figure 2: Illustration showing the intubation of the patient by a Foley’s catheter introduced adjacent to the rigid bronchoscope. |
The cuff of the catheter was then inflated distally to the lesion. The tip of the catheter was connected to a mechanical ventilator. The luminal diameter of the 18F catheter allowed a standard mechanical ventilator to the used. As the blood gas analysis was within normal ranges, jet ventilation was not required. Total mass excision and homeostasis were undertaken by cryosurgery. Video-1. Cryosurgery of an endotracheal mass through rigid bronchoscope accompanied by the usage of a Foley’s catheter as an intubation tube. http://cts.tgcd.org.tr/submit/pdf-files/in/532-CTS-532-8-video.mp4
The procedure was completed without any complications. The final pathology of the mass was granulomatous inflammation and necrosis. The patient was referred to the tuberculosis clinic for further medical treatment.
Written informed consent was obtained from the patient for publication of her data.
Discussion
In this case report, we described the use of Foley’s catheter as an effective way to facilitate the treatment of an undiagnosed semiocclusive endotracheal lesion with cryosurgery. Endobronchial tuberculosis (EBTB), which is a serious complication of pulmonary tuberculosis, has been reported in 10-40% of patients with active pulmonary tuberculosis [3,4]. These cases are often misdiagnosed as bronchial asthma or lung cancer [4]. Some patients with EBTB may have normal radiological findings (10–20%), and this may lead to a delay in the diagnosis [5]. Our patient was misdiagnosed with asthma and received medical therapy.The clinical features of EBTB are variable, and symptoms such as anorexia, weight loss, and night sweats might not be present in some cases. A progressive cough unresponsive to antitussive treatment is the most common presentation of EBTB. Sputum production is rare. Hemoptysis may occur, but it is rarely massive [6]. Computed tomography is a valuable diagnostic tool to differentiate EBTB from other bronchial lesions, such as bronchial stenosis and obstructions. A bronchoscopic examination is also important in the differential diagnosis to exclude other possible causes of EBTB-like symptoms [7,8].
EBTB may lead to serious complications, such as severe bronchostenosis, resistant tuberculosis, recurrent pulmonary infections, atelectasis, and bronchiectasis. It may even result in death due to respiratory failure and asphyxia [9,10]. Early diagnosis and antituberculosis treatment before involvement of the deeper airways are important to avoid sequelae of bronchostenosis [5].
Five potential mechanisms believed to be responsible for the development of EBTB are direct invasion from an adjacent parenchymal focus, implantation of organisms from infected sputum, hematogenous spread, erosion of a lymph node inside a bronchus, and lymphatic drainage from the parenchyma toward the peribronchial region [11]. In the present case, there was no parenchymal focus or an adjacent enlarged lymph node, and AFB staining of the sputum was negative.
EBTB is classified into seven subtypes according to its bronchoscopic findings: actively caseating, edema-hyperemic, fibrostenotic, tumor, granular, ulcerative, and nonspecific bronchic. Edematous-hyperemic, fibrostenotic, and tumorous subtypes tend to progress to eventual bronchial stenosis/obstruction within 3 months, despite appropriate treatment [12]. With these EBTB subtypes; sputum cultures, bronchial wash fluid, and biopsy samples typically show positive AFB staining or culturing results. The lesion in our case was similar to tumor-type EBTB. However, it differed from classical tumor-type EBTB because caseous material, usually seen in this type, was not observed [11].
We had no histopathologic diagnose of the endobronchial lesion at the time of the intervention. Noninvasive diagnostic techniques had not poined a current diagnosis. Although the patient had undergone medical treatment for a long time, her complaints had not regressed. The patient in the present case was a breast-feeding mother and had a low quality of life due to her ongoing bronchial symptoms. We did not perform FOB biopsy because of the risk of massive bleeding. Instead, cryosurgery was undertaken for both the diagnosis and treatment of the semiocclusive endotracheal tumoral lesion.
Cryosurgery can be used both in the diagnosis, debulking, and total excision of endobronchial lesions. As cryosurgery is minimally invasive and uses extreme cold to destroy lesions/tissue, and does not show any activity on cartilage; there is no risk of tissue damage or perforation. In addition, as cryosurgery has no risk of deflagration, the oxygen usage during the procedure is allowed freely. However, there are some risks of the application. Fragments separated from the mass and blood may move distal of the lesion and lead to airway obstruction and hypoxia. To prevent such complications, repeat aspiration of the distal airways is essential [13].
In our case, the location of the lesion just below the vocal cords caused difficulty in ventilation during rigid bronchoscopy. Such difficulty poses a potential medical emergency in the form of hypoxia. To prevent hypoxia, we had to secure the distal airway while maintaining ventilation. With this aim in mind, we used a soft Foley catheter for intubation. It was introduced adjacent to the RB. The balloon of the catheter was inflated distal to the lesion. The proximal tip of the catheter was then connected to a mechanical ventilator. In this way, we not only secured the distal airway but also enabled ventilation through the channel of the catheter. We also created a comfortable viewing and working space through the RB. The total operation time was 25 min. During the procedure, arterial blood gases analysis was conducted approximately every 10 min, and the highest levels of carbon dioxide and lowest levels of oxygen pressure were 55 mmHg and 100 mmHg, respectively.
In conclusion, endobronchial cryosurgery is a well-known treatment method for endobronchial occlusive lesions. The most challenging part of the intervention is securing the distal airway without causing hypoxia. With this in mind, in our case, we used a simple but facilitative technique comprising Foley’s catheter intubation close to a RB. To our knowledge, this method has not been reported elsewhere in the literature. We suggest that this new and simple method may be used to ensure the safety of the distal airways in endobronchial interventions and to achieve a comfortable viewing and working space through the RB in selected patients.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Reference
1) Shepherd RW, Radchenko C. Bronchoscopic ablation techniques in the management of lung cancer. Ann Transl Med. 2019;7:362.
2) Hautmann H, Gamarra F, Henke M, Diehm S, Huber RM. High frequency jet ventilation in interventional fiberoptic bronchoscopy. Anesth Analg 2000;90:1436-40.
3) Mu D, Nan D, LiW, Fu E, Xie Y, Liu T et al. Efficacy and safety of bronchoscopic cryotherapy for granular endobronchial tuberculosis. Respiration 2011;82:268-72.
4) Park MJ, Woo IS, Son JW, Lee SJ, Kim DG, Mo EK et al. Endobronchial tuberculosis with expectoration of tracheal cartilages. Eur Respir J 2000;15:800-2.
5) Um SW, Yoon YS, Lee SM, Yim JJ, Yoo CG, Chung HS et al. Predictors of persistent airway stenosis in patients with endobronchial tuberculosis. Int J Tuberc Lung Dis 2008;12:57-62.
6) Kashyap S, Solanki A. Challenges in endobronchial tuberculosis: from diagnosis to management. Pulm Med 2014;2014:594806.
7) Lee JH, Chung HS. Bronchoscopic, radiologic and pulmonary function evaluation of endobronchial tuberculosis. Respirology 2000;5:411-7.
8) Cakir E, Uyan ZS, OktemS, Karakoc F, Ersu R, Karadag B et al. Flexible bronchoscopy for diagnosis and follow up of childhood endobronchial tuberculosis. Pediatr Infect Dis J 2008;27:783-7.
9) Xue Q, Wang N, Xue X, Wang J. Endobronchial tuberculosis: an overview. Eur J Clin Microbiol Infect Dis 2011;30:1039-44.
10) Qingliang X, Jianxin W. Investigation of endobronchial tuberculosis diagnoses in 22 cases. Eur J Med Res 2010;15:309-13.
11) Kim EJ. A Tuberculosis-associated Endobronchial Polyp That Was Negative for Acid-fast Bacillus. Intern Med 2018;57:2701-4.
12) Siow WT, Lee P. Tracheobronchial tuberculosis: a clinical review. J Thorac Dis 2017;9:E71-7.






