2Department of Thoracic Surgery Yedikule Chest Diseases and Thoracic Surgery Training and Research Hospital, Istanbul, Turkey DOI : 10.26663/cts.2021.0022
Summary
Background: This study compared three commonly used scoring systems, the Acute Physiology and Chronic Health Evaluation II (APACHE II), Sequential Organ Failure Assessment (SOFA), and American Society of Anesthesiologists physical status classification (ASA), in the prediction of post-operative mortality and morbidity after lung resection.Materials and Methods: Adult patients admitted to the surgical intensive care unit (ICU) after lung resection between January 2018 and January 2020 were retrospectively evaluated.
Results: The study included 509 patients with a mean (SD) age of 59.9 (11.7) years; 421 of the patients were men (82.7%). The average ICU length of stay was 4.2 ± 6.9 days. Preoperative ASA scores were I-II in 73.5% and III-IV in 26.5% of the patients. The mean (SD) postoperative APACHE II score was 9.42 (4.23) and SOFA score was 1.04 (2.08). The area under the curve (AUC) in receiver operating characteristic analysis of postoperative complications was 0.772 for APACHE II and 0.690 for SOFA. The AUC values of APACHE II and SOFA scores for mortality were 0.925 and 0.944, respectively.
Conclusions: Our comparison of these scoring systems showed that SOFA was the best predictor of morbidity and mortality after lung resection. SOFA predicted the development of complications significantly better than both APACHE II and ASA. SOFA also predicted mortality better than ASA and APACHE II, although the difference was not significant for APACHE II. SOFA and APACHE II scores can be used to predict mortality and morbidity in patients lung resection surgery.
Introduction
Patients undergoing lung resection experience respiratory and cardiac problems that cause high perioperative morbidity and mortality. Despite advances in surgical technique and approach, the mortality rate ranges from 2% to 12%. The incidence of postoperative pulmonary complications is 19% to 59%, much higher than seen after upper abdominal (16-17%) or lower abdominal surgeries (0-5%). The total incidence of complications after thoracic surgery ranges between 15% and 37.5% [1-4].Physiological scoring systems have been developed for various purposes, including evaluating patients scheduled for surgery or admitted to the intensive care unit (ICU), documenting clinical observations and data, estimating the risk of mortality and morbidity, and making comparisons between patients or different ICUs. Scoring systems can also guide patient monitoring and treatment [5]. The Acute Physiology and Chronic Health Evaluation II (APACHE II), Sequential Organ Failure Assessment (SOFA), and American Society of Anesthesiologists physical status classification (ASA) are among the most commonly used scoring systems today.
The APACHE II model was developed in ICUs and applied in research and risk stratification of critical patients [6,7]. It yields a score ranging from 0 to 71 based on 12 physiological variables. This score can easily be calculated using routine data obtained in the first 24 hours after hospitalization, and the model is widely used in ICU populations.
The SOFA is a measure of multi-organ failure based on daily assessment of 6 organ systems: the respiratory, cardiovascular, coagulatory, renal, liver, and nervous systems. Each organ is rated from 0 (normal function) to 4 (high dysfunction/failure) to give a score of 0 to 24 points for the past 24 hours. SOFA scores enable the evaluation of changes in organ dysfunction or failure over time and can also be used to assess postoperative mortality and mobility [8-10].
In this study, it was aimed to compare the roles of APACHE II, SOFA and ASA in predicting postoperative mortality and morbidity of patients and to investigate the reasons why these scores were high.
Methods
This retrospective study included 509 consecutive adult patients admitted to the ICU after lung resection (lobectomy, pneumonectomy) between January 2018 and January 2020. The study flow chart is shown in figure 1.
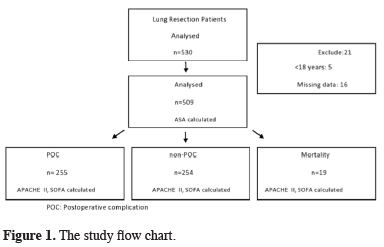 Click Here to Zoom |
Figure 1: The study flow chart. |
Indication for surgery was lung cancer in 453 (94.1%) of the patients and bronchiectasis in 56 (5.9%). A common general anesthesia protocol was used for all patients. Antibiotic prophylaxis was administered preoperatively. All surgical procedures were performed under general anesthesia after premedication with midazolam. Intravenous (IV) propofol 2-3 mg/kg and fentanyl 2 μg/kg were used for induction. As a muscle relaxant, 0.5 mg/kg IV rocuronium bromide was administered. A double-lumen endobronchial tube was placed on the right or left as appropriate and its position was confirmed by fiberoptic bronchoscopy (FBS). Anesthesia was maintained with 50% oxygen, 50% air, and 2% sevoflurane. Remifentanil IV infusion was continued throughout the operation. The surgical procedure was determined according to current guidelines for pneumonectomy and lobectomy. Resections were performed via thoracotomy or video-assisted thoracoscopic surgery (VATS). Emergency cases and cases that did not undergo pneumonectomy or lobectomy were not included in the study. Cardiac and respiratory complications occurred in 254 of the patients, while the other 255 did not develop any complications. Cardiac complications included arrhythmia, angina, myocardial infarction, and mortality. Respiratory complications included pneumonia, pulmonary edema, prolonged air leak, acute respiratory failure, and need for mechanical ventilation. The patients’ ASA scores were determined preoperatively and their APACHE II and SOFA scores were calculated based on the worst values recorded during their first day after admission to the ICU. The patients’ demographic data and follow-up information were also noted. All patients were transferred directly from the operating room to the ICU, then moved to the ward after a sufficient postoperative monitoring period. Patients who developed complications during follow-up in the ward were re-admitted to the ICU. Patients between the ages of 18-85 were included in the study, and patients with missing data were not included in the study.
Statistical Analysis
The patients’ demographic and clinical data were analyzed using IBM SPSS Statistics® version 23.0 (IBM Corp, Armonk, NY). Mean, standard deviation (SD), and minimum/maximum values were used as descriptive statistics for quantitative variables; frequency and percentage were used for qualitative variables. Normally distributed data were reported as mean (SD) and compared between groups using Student’s t-test. Qualitative variables were analyzed using Pearson’s chi-square test, or with Fisher’s exact test for small groups. Nonnormally distributed continuous variables were expressed as median and interquartile range (25th-75th percentiles) and compared using Mann-Whitney U test. Results with p values less than 0.05 were accepted as statistically significant. Diagnostic performance of APACHE II, SOFA, and ASA scores in predicting postoperative complications (POC) and mortality was examined using receiver operating characteristic (ROC) curves and area under the curve (AUC). Statistical analysis was performed using MedCalc Software for Windows to determine the best predictor. Multivariate logistic regression analysis was conducted using only variables shown to have a significant effect on POC and postoperative mortality in univariate analyses to identify independent risk factors. Spearman correlation analysis was used degree of relation between length of stay (LOS) and APACHE II and SOFA scores.
Results
A total of 509 lung resection patients, 421 (82.7%) male and 88 (17.3%) female, were included in this study. The mean age was 59.9 years. 374 (73.5%) of the patients were ASA I-II, 135 (26.5%) were ASA III-IV; 479 (94.1%) were lung cancer, 30 (5.9%) were bronchiectasis. Lobectomy was performed in 372 (73.1%) patients, pneumonectomy was performed in 137 (26.9%) patients, thoracotomy was performed in 399 (78.4%) and VATS type surgery was performed in 110 (21.6%) patients. The mean intensive care unit stay was 1.9 ± 1.7 days. The preoperative, peroperative and postoperative data of the patients in the study are shown in table 1.POC were observed in 255 patients (50.0%). Cardiac and respiratory complications were observed more frequently in complications. The mean APACHE II score of these patients was 10.94 ± 4.87 and the SOFA score was 1.82 ± 2.65. Of the 135 patients with SOFA score ≥ 2 and APACHE score ≥ 10, cardiac complications predominated in 69 (51%) and respiratory complications in 66 (49%). While cardiac complications were observed in 123 (90%) arrhythmia, 9 (6.6%) heart failure, 8 (5.9%) angina and 5 (3.7%) myocardial infarction; respiratory complications were listed as acute respiratory failure 44 (35%), pneumonia 62 (50%), pulmonary edema 10 (7%) and pulmonary embolism 4 (6%). Of the patients who developed PC, 31.5% (n = 39) required invasive mechanical intervention and 40.3% (n = 50) underwent CPAP.
The mean age was 62.5 ± 9.7 years in patients who developed POC, and 65.4 ± 8.9 years in those who developed mortality. Factors associated with POC development were male sex, advanced age, high ASA (III/IV), APACHE II, and SOFA scores, resection due to lung cancer, and pneumonectomy (p < 0.001 for all). The other variables did not differ significantly between patients with and without POC. Patients with POC had statistically longer ICU LOS (mean 2.9 ± 2.0 days vs. 1.0 ± 0.0 day, p < 0.001).
Of the 19 patients who died during the study period, 12 had respiratory complications and 7 had cardiac complications. The mean APACHE II score of these patients was 19.63 and the SOFA score was 6.89. Mortality was associated with advanced age (p = 0.007), high APACHE II and SOFA scores (p < 0.001 for both), and pneumonectomy (p < 0.001). The other variables showed no significant differences between surviving and ex patients. ICU LOS was statistically longer among nonsurvivors (mean 5.0 days versus 1.8 days, p < 0.001).
ROC analysis of SOFA, APACHE II, and ASA scores’ relationship with POC and mortality revealed that all three scoring systems were significantly effective in predicting POC. However, only SOFA and APACHE II scores were significant predictors of mortality (Table 2). The ROC curves for SOFA and APACHE are shown in figures 2 and 3.
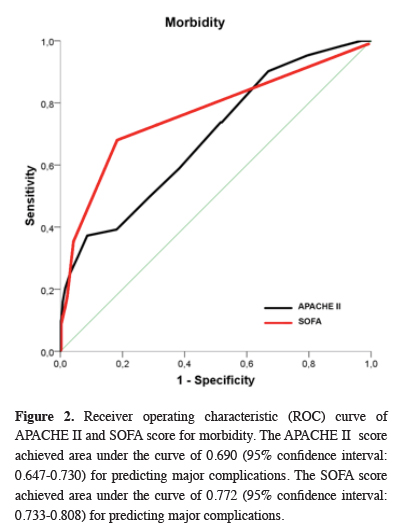 Click Here to Zoom |
Figure 2: Receiver operating characteristic (ROC) curve of APACHE II and SOFA score for morbidity. The APACHE II score achieved area under the curve of 0.690 (95% confidence interval: 0.647-0.730) for predicting major complications. The SOFA score achieved area under the curve of 0.772 (95% confidence interval: 0.733-0.808) for predicting major complications. |
 Click Here to Zoom |
Figure 3: Receiver operating characteristic (ROC) curve of APACHE II and SOFA score for mortality. The APACHE II score achieved area under the curve of 0.925 (95% confidence interval: 0.898-0.946) for predicting mortality. The SOFA score achieved area under the curve of 0.944 (95% confidence interval: 0.920-0.962) for predicting death. |
Comparison among the scoring systems showed that SOFA was the best predictor of POC and mortality (Table 2).
SOFA predicted POC development significantly better than both APACHE II (p = 0.002) and ASA (p < 0.001). While SOFA predicted mortality better than APACHE II, this difference was not significant (p = 0.502). APACHE II also predicted POC development better than ASA, but not significantly (p = 0.318). However, APACHE II predicted mortality significantly better than ASA (p < 0.001).
In ROC analysis, a SOFA score of 1 was identified as the optimal cut-off for prediction of POC. Comparison of patients with SOFA ≥ 1 (n = 221) and those with SOFA scores <1 (n = 288) revealed that the high SOFA group had a statistically significantly higher rate of POC than the low SOFA group (79.2% vs. 27.8%, p < 0.0001; odds ratio [OR]: 9.891, 95% CI: 6.534-14.974).
For postoperative mortality, the optimal SOFA cut-off was 2. Comparison of patients with SOFA ≥ 2 (n = 408) and low SOFA < 2 (n = 101) showed that the high SOFA group had statistically higher postoperative mortality (17.8% vs. 0.2%, p < 0.0001; OR: 88.265, 95% CI: 11.622-670.353).
The cut-off score for APACHE II in the prediction of POC was 12. Patients with APACHE II ≥ 12 (n = 392) had a significantly higher POC rate compared to patients with APACHE II score < 12 (n = 117) (81.2% vs. 40.8%, p = 0.0001; OR: 6.261, 95% CI: 3.777-10.381).
An APACHE II score of 13 was the optimal cut-off for postoperative mortality. The mortality rate was significantly higher among patients with APACHE II scores ≥ 13 (n = 91) than those with APACHE II score < 13 (n = 418) (18.7% vs. 0.5%, p < 0.0001; OR: 47.784, 95% CI: 10.814-211.150).
In multivariate analysis using the independent variables that were significantly associated with POC development (Table 1) and the SOFA ≥ 1 and APACHE II ≥ 12 groups, we found that sex, ASA III/IV, resection type, high APACHE II, and high SOFA were found to be independent risk factors for POC (Table 3).
Table 3: Multivariate Logistic Regression Analysis for Postoperative Complications.
In the multivariate analysis including independent variables associated with mortality (Table 1) and the SOFA ≥ 2 and APACHE ≥ 13 groups identified in ROC analysis, we determined that resection type, high APACHE II, and high SOFA were independent risk factors for postoperative mortality (Table 4).
Table 4: Multivariate logistic regression analysis for postoperative mortality.
There was a strong positive correlation between LOS and SOFA score (r = 0.530, p < 0.001) and a moderate positive correlation between LOS and APACHE II score (r = 0.330, p < 0.001).
Discussion
In this study, we evaluated the ability of the SOFA and APACHE II scoring systems to predict mortality and morbidity after lung resection. The SOFA and APACHE scores are the most commonly used tools for the assessment of mortality and morbidity in non-surgical ICUs [11]. According to our study results, APACHE II and SOFA may be acceptable scoring systems for predicting hospital mortality and morbidity after lung resection.In a study of 600 patients admitted to the ICU after cardiac surgery, APACHE II score was found to be a useful method for predicting mortality, with the best predictive ability at scores above 9 (AUC = 0.743) [6]. In our study, both SOFA and APACHE II scores significantly predicted mortality. We found that the APACHE II was a good predictor of mortality in patients undergoing lung surgery, with an AUC of 0.925 and an optimal cut-off of ≥ 12. Similarly, SOFA score had an AUC of 0.944 and a cut-off of > 1. In our study population, the median APACHE II score was 10.94 in those with POC and 7.78 in those without POC. The prevalence of POC among patients with high APACHE II scores (≥ 12) was 81.2%, compared to 40.8% in those with low scores (< 12). In other words, APACHE II scores above the cut-off value were associated with a two-fold increase in morbidity.
High APACHE II scores are an indicator of higher mortality. In a study of 8515 ICU patients, Knaus et al reported that APACHE II scores were between 20 and 35 for all nonsurgical patients, while mortality was 40% to 75% [12]. Ulus et al reported an ICU mortality rate of 44.8% among patients whose APACHE II scores were 20 to 35 at ICU admission [13]. APACHE II score was found to be useful in predicting post-ICU mortality and readmission in surgical patients [14]. In our study, the mortality rate was 0.5% in patients with APACHI II <13, while it was 13% in patients with >13.
In their study of 1190 intensive care patients, Lee et al stated that the APACHE II score is a useful scoring system for both admission to intensive care and discharge [13]. In our study, the duration of intensive care hospitalization (LOS) was 1 day in the group that did not develop complications, while the average in the group that developed complications was 2.9 days. In the group that developed mortality, the average duration of hospitalization was observed as 5 days. Mortality with LOS was statistically increased in those who developed it (5.0 days versus 1.8 days on average). Again, a statistically significant association was observed between the high SOFA score and LOS.
The SOFA system was developed at a consensus meeting of the European Society of Intensive Care Medicine in 1994 and further revised in 1996 [15]. A study evaluating BNP, Euroscore, and SOFA scores in cardiac surgery patients showed that SOFA score was useful in predicting mortality [16]. In a study including 272 lung transplant patients, the mean SOFA and APACHE II scores of the 244 surviving patients were 17.4 and 4.9, while those of the nonsurviving 28 patients were 24.2 and 7.1, respectively [17]. In our study, there was a difference between SOFA score ≥ 2 and SOFA score < 2 in terms of postoperative mortality in terms of 17.8% versus 0.2%. An increase in mortality was observed as the SOFA score increased.
In the present study, we observed that SOFA was better able to predict POC and mortality compared to ASA and APACHE II. Mortality prediction was better with SOFA than APACHE II, although the difference was not statistically significant. Furthermore, the rate of POC was 79.2% among patients with SOFA scores ≥ 1, compared to 27.8% at values < 1. In other words, patients with a SOFA score of 1 or higher had a 9.8-fold higher risk of developing POC compared to those with a score below 1. SOFA predicted POC development significantly better than both APACHE II and ASA. APACHE II predicted mortality development significantly better than ASA.
ASA score is used for preoperative patient assessment and is useful in determining the anesthetic approach, especially in terms of monitoring methods. It is widely accepted and applied worldwide. The system was endorsed by the American Society of Anesthesiologists in 1940 to determine surgical risk. Higher scores are associated with increased perioperative mortality and morbidity. We observed that ASA III-IV was a significant predictor of POC compared to ASA I-II, consistent with the results of similar studies [18,19].
Despite advances in lung surgery, mortality and morbidity rates are still high [20-22]. The frequency of POC is affected by factors such as resection type, patient demographic characteristics, procedure duration, and intraoperative bleeding. Age, gender, renal dysfunction, hypertension, chronic8 obstructive pulmonary disease (COPD), and lymph node dissection are considered risk precursors for cardiopulmonary complications [5,6]. In our study, male sex, advanced age, high ASA (ASA III/IV) score, lung cancer-induced resection, high APACHE II and SOFA score, and pneumonectomy were negatively affected by the development of complications. Markers of mortality after lung cancer resection are listed as BMI, male sex, renal dysfunction, chemotherapy, pneumonectomy, bilobectomy, and emergency surgery [3,11]. In our study, advanced age, high APACHE II and SOFA scores, and pneumonectomy were negatively affected the development of mortality. In the study of factors affecting APACHE II and SOFA scores higher than their normal levels, arrhythmias in cardiac complications, pneumonia and acute respiratory failure were observed to be prominent in respiratory complications.
The main limitations of this study are the small patient group and its retrospective design. Furthermore, although it was a single-center study, the surgeries were performed by different surgeons.
In conclusion comparison of these three scoring systems demonstrated that SOFA was the best predictor of morbidity and mortality after lung resection. SOFA predicted both complications and mortality significantly better than ASA and predicted complications significantly better than APACHE II. Prediction of mortality was also better compared to APACHE II, but the difference was not significant.
Scoring systems are an important tool in pulmonary surgery practice that evaluate operative mortality and morbidity. Validation studies are needed to predict and evaluate complications and mortality observed after lung resection. According to the findings of this study, SOFA and APACHE II scores had high AUC values for predicting mortality (0.944 and 0.925, respectively) and moderate AUC values for predicting morbidity (0.772 and 0.690, respectively). These results indicate that patients with high APACHE II and SOFA scores should be monitored more closely to reduce the incidence of mortality and morbidity. However, our study was conducted with a limited number of patients and the results should be confirmed in larger studies.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Ethics Approval
The study was approved by the institutional ethical board of Health Sciences University, Istanbul Yedikule Chest Disease and Thoracic Surgery Training and Research Hospital (No: 271-05/23.08.2020).
Authors’ contributions
YÖ, CA; co-wrote the paper, collected the data, performed the analysis, contributed data/analysis tools, conceived and designed the analysis.
Reference
1) Stéphan F, Boucheseiche S, Hollande J, Flahault A, Cheffi A, Bazelly B et al. Pulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factors. Chest 2000; 118:1263-70.
2) De Decker K, Jorens PG, Van Schil P. Cardiac complications after noncardiac thoracic surgery: an evidence-based current review. Ann Thorac Surg 2003; 75: 1340-8.
3) Rena O, Papalia E, Oliaro A, Casadio C, Ruffini E, Filosso PL et al. Supraventricular arrhythmias after resection surgery of the lung. Eur J Cardiothorac Surg 2001; 20: 688-93.
4) Algar FJ, Alvarez A, Salvatierra A, Baamonde C, Aranda JL, López-Pujol FJ. Predicting pulmonary complications after pneumonectomy for lung cancer. Eur J Cardiothorac Surg 2003; 23: 201-8.
5) Cakir E, Sari E, Bindal A, Çiftçi A, Özkoçak Turan I. Comparison of the Effectiveness of Acute Physiology and Chronic Health Evaluation II and Modified Early Warning Score Scoring Systems in Predicting Mortality in Patients in the Intensive Care Unit. Yoğun Bakım Derg 2019; 10: 75-9.
6) Yalçın M, Gödekmerdan E, Tayfur K, Yazman S, Ürkmez M, Ata Y. The APACHE II Score as a Predictor of Mortality After Open Heart Surgery. Turk J Anaesthesiol Reanim 2019; 47: 41-7.
7) Wang L, Chen R, Mo Z, Dong J, Sun Z, Xiao F et al. Predictive value of SOFA score combined AGI grading system in elderly patients with sepsis: a retrospective analysis with 91 patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2017; 29: 145-9.
8) Aldrich JM, Gropper MA. Can we predict pulmonary complications after thoracic surgery? Anesth Analg 2010; 110: 1261-3.
9) Pätilä T, Kukkonen S, Vento A, Pettilä V, Suojaranta-Ylinen R. Relation of the Sequential Organ Failure Assessment score to morbidity and mortality after cardiac surgery. Ann Thorac Surg 2006; 82: 2072-8.
10) Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707-10.
11) Geyik FD, Altun GT, Çıtak N. A Comparıson Of Apache II And Apache IV Scorıng Systems in Patients Admitted to an Intensive Care Unit. Journal of Anesthesia – Jarss 2013; 21: 182-6.
12) Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818-29.
13) Ulus F, Kokulu S, Savkılıoğlu E. Survıval Analysıs Of The Elderly Patıents Treated In The Respıratory Intensıve Care Unıt Turkish Journal Of Geriatrics 2010; 13: 231-7.
14) Lee H, Lim CW, Hong HP, Ju JW, Jeon YT, Hwang JW et al. Efficacy of the APACHE II score at ICU discharge in predicting post-ICU mortality and ICU readmission in critically ill surgical patients. Anaesth Intensive Care 2015; 43: 175-86.
15) Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med 2009; 37: 1649-54.
16) Kartufan F, Karaoğlu K. Mortality after Cardiac Surgery; a Comparison of BNP, EuroSCORE and SOFA Score. GKDA 2018; 24: 111-7.
17) Rello J, Bello I, de Vicente R, Hermira Anchuelo A, Ballesteros MÁ, Iranzo R et al. EMPRET Study investigators. Risk Factors for Mortality in 272 Patients With Lung Transplant: A Multicenter Analysis of 7 Intensive Care Units. Arch Bronconeumol 2017; 53: 421-6.
18) Curatolo C, Goldberg A, Maerz D, Lin HM, Shah H, Trinh M. ASA physical status assignment by non-anesthesia providers: Do surgeons consistently downgrade the ASA score preoperatively? J Clin Anesth 2017; 38: 123-8.
19) Ozkan E, Fersahoğlu MM, Dulundu E, Ozel Y, Yıldız MK, Topaloğlu U. Factors affecting mortality and morbidity in emergency abdominal surgery in geriatric patients. Ulus Travma Acil Cerrahi Derg 2010; 16: 439-44.
20) Çınar HU, Kocatürk C, Cansever L, Ceyhan S, Bedirhan MA. Morbidity, mortality and survival rates of non-small cell lung cancer patients who underwent lobectomy with pulmonary artery reconstruction compared to those of the patients who underwent pneumonectomy. Curr Thorac Surg 2020; 5: 1-9.






