2Yedikule Teaching Hospital for Chest Diseases and Thoracic Surgery, Department of Pathology, Istanbul, Turkey. DOI : 10.26663/cts.2017.0006
Summary
Pulmonary metastases of papillary thyroid carcinoma (PTC) is rare and generally present as a micronodular or a miliary pattern. Herein we present a single giant chest wall metastasis of PTC that caused mediastinal shift which was not reported in the literature. A 67 year old female patient admitted to the hospital due to shortness of breath. A right sided mass on the chest wall and thyroid nodules were detected. PTC was diagnosed and the patient underwent a total thyroidectomy. pT3N0 were evaluated postoperatively. PETCT demonstrated the mass at right hemithorax (12 cm lesion, SUV max=7.2) plus needle biopsy showed a metastasis of PTC. A chest wall resection plus right upper lobectomy and lymph node dissection was performed as the first choice for PTC metastasis.Introduction
Cancer from almost every organ in the body can metastasize to the lung such as colon, pancreas and breast carcinoma. Metastatic lung nodules can be found alone or in groups [1]. Pulmonary metastases of papillary thyroid carcinoma (PTC) is rare and usually has a micronodular or miliary pattern [2]. In this article, we report a case which presented with a single giant chest wall metastasis that arose from PTC and causing a mediastinal shift.Case Presentation
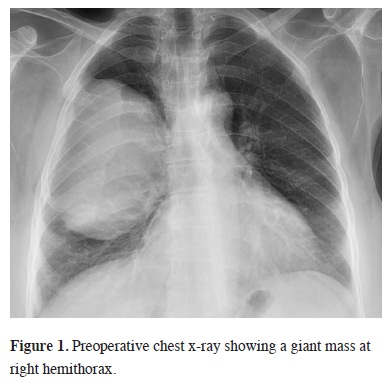
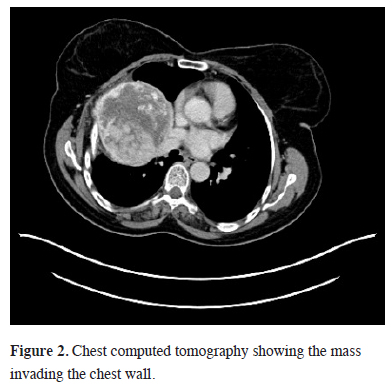
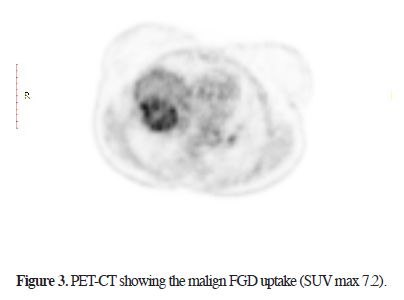
Sixteen months ago, a 67-year-old female patient, applied to another hospital due to shortness of breath. A mass at the right hemithorax was found as a result of investigations. Diagnostic transthoracic needle aspiration was performed without providing a diagnosis. Positron emission Tomography (PET-CT) was performed due to the suspicion of malignancy. A mass filling 60% of the right hemithorax (SUV max 5.4) and a nodule in the left thyroid lobe (SUV max 6.0) were found. Histopathologic diagnosis from the biopsy of the thyroid nodule revealed the presence of PTC and treatment options were discussed in a multidisciplinary council and thyroid surgery was planned. The final pathological results of patients who underwent total thyroidectomy confirmed PTC without a capsular invasion (pT3N0). No other additional treatment options for the thyroid lesion were presented at the same council. A thoracic surgical intervention for the chest wall mass was refused by the family. The patient was referred to our hospital. The patient"s posterioanterior (PA) chest x-ray and chest computed tomography showed a thoracic mass with chest wall invasion (Figures 1, 2). The lesion - measured approximately 12 cm in diameter - caused a mediastinal shift, and a rare calcifications were detected. Needle biopsy from the mass confirmed the diagnosis of papillary thyroid carcinoma metastasis. Cranial MRI showed no brain metastasis. A new PET/CT showed a higher SUV max as 7.2 compared to the previous study without any distant organ metastasis (Figure 3).
 Click Here to Zoom |
Figure 1: Preoperative chest x-ray showing a giant mass at right hemithorax. |
 Click Here to Zoom |
Figure 2: Chest computed tomography showing the mass invading the chest wall. |
 Click Here to Zoom |
Figure 3: PET-CT showing the malign FGD uptake (SUV max 7.2). |
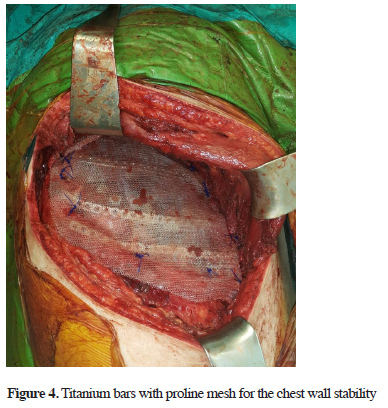
The mass (12.5x10x6.5 cm) was totally excised with partial resection of the invaded right 2nd, 3rd, 4th, and 5th ribs. A lobectomy due to parenchymal destruction and lymph node dissection (4th, 7th, 9th, 10th, and 11th lymph nodes) was also performed. The large chest wall defect was closed using 3 titanium bars and a proline mesh (Figure 4). There were no complications from the procedure and the patient was discharged at day 5 uneventfully.
 Click Here to Zoom |
Figure 4: Titanium bars with proline mesh for the chest wall stability |
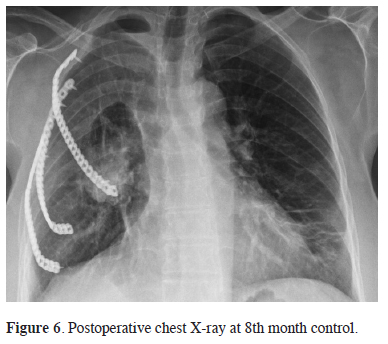
The final histopathological examination confirmed the diagnosis of PTC metastasis. The surgical margins was clear and, lung parenchyma and lymph nodes did not show any evidence of tumor cells. The diagnosis was supported by immunostaining (Thyroglobulin (CM022C) and HB -1 was positive) (Figure 5). Radioactive iodine (I131) was provided as an additional treatment to the patient. At the 8th month control no recurrences was seen (Figure 6).
 Click Here to Zoom |
Figure 5: Pathological examination: immunostaining (Thyroglobulin (CM022C) positive, the HB -1 positive) |
 Click Here to Zoom |
Figure 6: Postoperative chest X-ray at 8th month control. |
Discussion
Thyroid cancer constitutes 1% of all cancers [3]. The incidence of thyroid cancer is 5.5 to 6.6%, and is the fastest growing type of malignancy in both sexes. Ten year survival is 98% after surgery for PTC. However recurrence of thyroid cancer is most commonly seen with the this subtype [4]. Recurrence after surgery was reported as 24% [5] at a Tubiana study. Therefore, close follow-up of patients was recommended.Bone metastases with the follicular type and PTC occur 7-20% and 1-7% of the cases respectively. Medical drugs (analgesics, bisphosphonates), radiotherapy, surgery and radioisotopes are used in a multidisciplinary approach for the treatment of thyroid metastasis. I131 is an effective treatment modality for treatment of thyroid cancer with bone metastasis however resistance is frequently observed. Surgical treatment is indicated when patients present with intractable pain, lesions are unresponsive to medical therapy, there is low radioactive involvement, or present with spinal instability [6]. While the first option in the treatment of metastases is usually I131 therapy, we suggested surgery as a more viable option because of complaints of dyspnea, the presence of a mediastinal shift, and compression of the mass in our case. However, we did use I131 in the post-operative treatment of our patient.
A literature search was carried out in "PubMed" and "ResearchGate" using the following keywords: papillary thyroid carcinoma, chest wall and metastasis. Four studies were found that resemble to our current case. Kaya and Çermik [7] reported a case with diagnosis of papillary carcinoma metastasis who underwent surgery but died due to multiple new metastases. In the present case, a metastatic lesion was also detected first, but it was quite large and single so we anticipated that it would have a relatively good prognosis. In the second case in the literature, presented by Li et al. [8] a chest wall surgery was performed using endoscopic thyroid surgery with metastasis to the sternocleidomastoid muscle. Our patient had distant metastases independent of the remote operation. Nakada et al. [9] presented the third case. After PTCs, the sternal metastases became resistance to radioactive iodine therapy; therefore, percutaneous ethanol injection (PEI) therapy was successfully performed. In our case, many of the unknown effects of PEI on dyspnea and due to the emergency situation caused by the mediastinal shift, "surgery" was considered as the first choice of treatment. Karamustafaoğlu et al. [10] presented the fourth case report having similarities to our case. In this series of 4 patients, 3 patients presented PTC and one diagnosed as medullary thyroid carcinoma. Three patients who underwent chest wall resection died due to distant metastasis between 5 to 36 months, but one PTC case was still alive at 5th year follow-up. We closely monitored our patient up to the 8th month because of recurrence rate.
As a conclusion and take home message papillary thyroid carcinoma should be closely monitored after surgery, even if the malignant potential of thyroid carcinoma is low. For the treatment of metastases radioactive iodine treatment, as the first choice, should be kept in mind however when a resistance is faced surgical treatment could be performed. In the cases with a mediastinal shift and dyspnea caused by a resectable tumor, surgery should be considered as the first choice.
Declaration of conflicting interests
The author declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The author received no financial support for the research and/or authorship of this article.
Reference
1) Stern EJ, Swensen SJ, Kanne JP. High-resolution CT of the Chest, 3rd Ed: Wolters Kluwer, Philadelphia PA; 2010.
2) Kaseda K , Watanabe K , Sakamaki H, Kazama A. Solitary pulmonary metastasis from occult papillary thyroid carcinoma. Thoracic Cancer 2016; 7: 261-3.
3) Sherma SI. Thyroid carcinoma. The Lancet 2003; 361: 501-11.
4) Kim M, Ladenson PW: Thyroid. In: Goldman L, Schafer AI (eds). Goldman-Cecil Medicine, 25th Ed. Philadelphia PA: Elseiver; 2016:1513.
5) Tubiana M, Schlumberger M, Rougier P, Laplanche A, Benhamou E, Gardet P, et al. Long-term results and prognostic factors in patient with differentiated thyroid carcinoma. Cancer 1985; 55: 794-804.
6) Muresan MM, Olivier P, Leclère J, Sirveaux F, Brunaud L, Klein M, et al. Bone metastases from differentiated thyroid carcinoma. Endocr Relat Cancer 2008; 15: 37-49.
7) Kaya M, Cermik TF. Tc-99m MIBI scintigraphy in tall cell variant of papillary thyroid carcinoma with negative radioiodine scan. Clin Nucl Med 2008; 33: 615-8.
8) Li S, Zhang F, Zhang Y, Liang Y, Qi X, Yang X, Jiang J. Implantation at sternocleidomastoid and chest wall after endoscopic thyroid carcinoma surgery. Surg Laparosc Endosc Percutan Tech 2012; 22: 239-42.






