2Department of Thoracic Surgery, Ataturk Chest Disease and Thoracic Surgery Training and Research Hospital, Ankara, Turkey DOI : 10.26663/cts.2022.023
Summary
Background: In parallel with the change in circulating tumor cells, the no-touch isolation technique is offered as an option to reduce recurrences of non-small-cell lung cancer. We aimed to examine the relationship of this technique with recurrence and survival in our clinic.Materials and Methods: Among 675 patients who were operated on with the diagnosis of lung cancer between 2009 and 2015, 98 patients with tumor size of less than 3 cm in the postoperative pathology report, no visceral pleural invasion or lymph node involvement, and a negative surgical margin were included in the study. The patients were divided into two groups as patients treated with and without the no-touch isolation technique (i.e., a wedge resection group prior to lobectomy and a direct lobectomy group), and the results of recurrence and survival were evaluated statistically.
Results: While adenocarcinoma was observed more frequently in the wedge resection group, squamous cell carcinoma was observed statistically more frequently among patients treated with direct lobectomy (p < 0.001). There was no statistically significant difference in recurrence or survival rates between patients treated with and without the no-touch isolation technique (p = 0.746 and p = 0.689, respectively).
Conclusions: Although wedge resection before surgery is theoretically well grounded, we found that it was not clinically significant as a result of our study. The technique may prove beneficial in reevaluating chemotherapy indications based on circulating tumor cells, especially in early-stage cases where patients have not received chemotherapy, and prospective studies are needed in this regard.
Introduction
For patients with early-stage non-small-cell lung cancer (NSCLC), the primary treatment option remains lymph node dissection combined with anatomic lung resection despite alternative treatment approaches recently proposed [1]. Nevertheless, local recurrence or distant metastasis is observed in 30% of these cases and the 5-year survival rate is only 66-82% [2]. Evidence suggests that circulating tumor cells (CTCs) may be the leading cause of recurrence and distant metastasis, and studies have associated the presence of CTCs with significantly low survival rates [3-5]The main surgical principle to prevent recurrence and distant metastasis is ligating the pulmonary vein before the artery during malignancy surgery [6]. However, the lobe is still manipulated during dissection of the pulmonary vein from the hilar structures and its rotation, potentially causing higher numbers of CTCs. Some authors argue that the CTC burden can be reduced by the no-touch isolation technique (NTIT), which involves the wedge resection of tumor-bearing tissue before the planned anatomical resection [2]. The technique has also yielded significant results in colorectal carcinoma and other non-pulmonary malignancy surgeries [7,8].
Thanks to protective mechanisms such as oxidative stress and immune response, the presence of CTCs in peripheral blood is not directly linked to their migration to distant tissues causing metastases [9]. However, a limited number of recent studies have reported significantly reduced recurrence after surgical resections with NTIT [6,7].
Here, we retrospectively compare the results of recurrence and survival among early-stage NSCLC patients who underwent lobectomy after NTIT or direct lobectomy.
Methods
Patient dataThe initial population consisted of 675 patients operated on for NSCLC in our clinic between 2009 and 2015. We excluded patients with tumor size of >3 cm, non-anatomical resection, perioperative chemotherapy and radiotherapy, visceral pleural and lymph node invasion, or a positive surgical margin. As a result, the study included 98 NSCLC patients without indication for chemotherapy, who were followed with no postoperative treatment and had tumor size of <3 cm with no lymph node or visceral pleural invasion and a negative surgical margin.
The lobe was removed with the help of an endobag for all patients who were operated on with video-assisted thoracoscopic surgery (VATS). In addition, while performing lung resection, vein ligation was performed before the artery, adhering to standard surgical principles.
The patients were divided into two groups as the NTIT group treated with wedge resection before lobectomy and the non-NTIT group treated with direct lobectomy. In our clinical approach, intraoperative wedge resection is performed for patients who cannot be diagnosed with preoperative biopsy and a frozen sample is studied, while patients with a preoperative diagnosis directly undergo anatomic lung resection. The non-NTIT group included patients diagnosed with preoperative methods (transthoracic biopsy, bronchoscopy). We evaluated patient parameters such as age, gender, tumor size, tumor subtype (adenocarcinoma or squamous cell carcinoma), type of surgery, recurrence, time to recurrence, and survival, as well as factors affecting recurrence and survival. Local ethics committee approval was obtained (2012-KAEK-12/2399).
Statistical Analysis
The SPSS 22.0 statistical package program was used for the statistical analysis of data. Categorical variables were presented as number and percentage, while continuous variables were shown as mean ± standard deviation if normally distributed and as median (min-max) if not normally distributed. Pearson’s chi-square test, Fisher’s exact test, and the chi-square test with Yates continuity correction were used to compare categorical variables between independent groups. The normality of continuous variables was evaluated using visual methods (histogram and probability plot) as well as analytical methods (Kolmogorov-Smirnov and Shapiro-Wilk tests). Our analysis revealed that the continuous variables were not normally distributed. We used the Mann-Whitney U test to compare the two groups for the other variables. The Kaplan-Meier method was used to calculate estimated survival and the patients who died from other causes were censored. Differences in survival rates were assessed using the log-rank test with regard to gender, presence of wedge resection, pathological subtype, and recurrence.
Results
Of the 98 patients in our study, 42 underwent wedge resection before anatomic resection (NTIT group) and 56 underwent direct anatomic resection (non-NTIT group). All wedge resections were performed with VATS, while anatomical lung resections were performed with VATS for 17 patients and thoracotomy for 81 patients. The anatomical resections included lobectomy (n = 84), bilobectomy (n = 6), pneumonectomy (n = 5), segmentectomy (n = 2), and sleeve lobectomy (n = 1). Table 1 shows the patients’ ages, gender distribution, tumor sizes, pathological tumor subtypes, recurrence rates, and time to recurrence by groups. We observed a significant difference between the groups in terms of tumor subtype. Adenocarcinoma cases were significantly more frequent in the NTIT group (27/15) than the non-NTIT group (17/56), whereas squamous cell carcinoma was significantly more frequent in the non-NTIT group (39/56) than the NTIT group (15/42) (p < 0.001).Table 1: Patients’ characteristics and results of the analysis.
Table 2: Survival analysis of the patients according to covariates.
Similarly, there was no significant difference between the groups regarding factors affecting survival. We detected no significant differences in recurrence, tumor subtype, gender, or survival (Table 2). The 5-year overall survival rate was 78.6% and the 10-year survival rate was 76.1%.
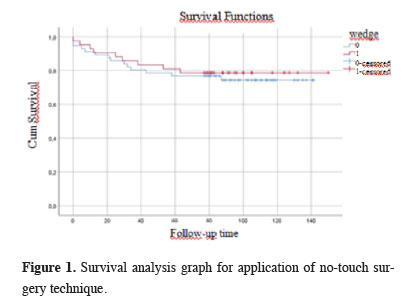
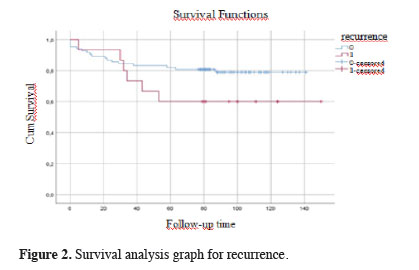
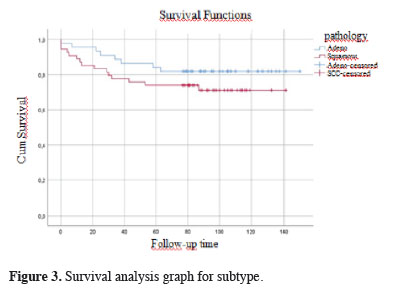
Figures 1, 2, and 3 show the survival graphs by recurrence, tumor subtype, and application of NTIT.
 Click Here to Zoom |
Figure 1: Survival analysis graph for application of no-touch surgery technique. |
 Click Here to Zoom |
Figure 2: Survival analysis graph for recurrence. |
 Click Here to Zoom |
Figure 3: Survival analysis graph for subtype. |
Discussion
Our investigation revealed no relation between application of NTIT, which involves the removal of the tumor together with the surrounding parenchymal tissue, i.e., wedge resection, before anatomical resection, and recurrence (p = 0.746). Similarly, we observed no significant link between pathological tumor subtype, age, gender, or the application of NTIT and survival (p = 0.689). The use of NTIT is based on the fact that the manipulation of the lung lobe containing the tumor leads to an elevated level of CTCs. Numerous retrospective and prospective studies on CTCs in the literature have reported increased numbers of CTCs resulting from tumor manipulation, and especially in advanced lung cancer patients, which is, in turn, associated with higher recurrence and lower disease-free survival rates [10-13]. Evidence has also shown the role of CTCs in other cancer types besides lung cancer [14,15].Yasukawa et al [6,7] evaluated the NTIT approach and reported its efficacy, but the breadth of inclusion criteria in their research rendered their results controversial. As is widely known, specific guidelines are available for chemotherapy indications in NSCLC. One parameter in these guidelines is pleural invasion, and the others are tumor size and lymph node involvement. Since cancer is considered systemic, the presence of these parameters strengthens this feature of the disease, making a case for the administration of adjuvant chemotherapy [16-19]. In one study, Yasukawa et al included tumors of all sizes [7]; in another study of them, they included tumors of <3 cm, but their findings are questionable because they did not exclude factors affecting the tumor stage, such as visceral pleural invasion. In contrast, the present study involved T1N0M0 (stage 1) patients without indications for chemotherapy, and all parameters influencing tumor stage, such as pleural invasion, were considered as exclusion criteria. The 5-year overall survival rate was 78.6% and the 10-year survival rate was 76.1%. It is thought that the reason for these high survival rates is the application of the exclusion criteria. However, due to the number of cases, further evaluations could not be made.
A previous study indicated the sensitivity of CTC detection in NSCLC to be 100% for stages 2-4 and about 50% for stage 1 patients. In addition, the specificity of CTC detection was almost 100%, whereas its sensitivity varied considerably depending on the method used [20,21]. Other studies on other tumor types have reported reduced circulating tumor DNA (ctDNA) levels for lower disease stages [14,22]. Diehl et al [23] and other authors investigating non-pulmonary malignancies [24] also showed decreased CTC levels after surgical resection, associating continued high levels with increased recurrence rates.
On the other hand, there is an ongoing debate as to whether a direct correlation exists between the presence of CTCs and clinical outcomes [25]. Recent improvements have allowed tumor diagnosis with liquid biopsy; still, not all tumors result in recurrence or distant metastasis, thanks to the body’s various defense responses. In this regard, macrophage activity has been emphasized for CTCs. Other primary markers for tumor localization include tumor infiltration and compliance of host cells [26-30]. Therefore, prospective studies of large series limited to early-stage lung cancer cases without indications for chemotherapy are necessary to determine the clinical efficacy of NTIT decisively.
NTIT was significantly more frequently performed among adenocarcinoma cases than cases of squamous cell carcinoma in our cohort (p < 0.001). We consider the relatively central location of squamous cell carcinoma and the high diagnostic accuracy of preoperative bronchoscopy as the principal reasons for this finding. Yasukawa et al. also indicated a significantly higher rate of peripheral tumor localization in their wedge resection group [6]. In the practice of our clinic, bronchoscopy is routinely applied in preoperative evaluations before malignancy surgery. Therefore, the rate of preoperative diagnosis is high in cases of central tumors, and especially for the squamous cell carcinoma subtype. However, if there is a high suspicion of malignancy in peripherally located tumors in preoperative PET results, surgical resection will be planned for patients who are suitable for surgery whether or not the transthoracic biopsy result is diagnostic, and our clinical approach is to perform a diagnostic procedure with an intraoperative frozen section. For this reason, we frequently use surgical diagnostic methods for patients who are candidates for surgical resection and we think that this approach played a role in achieving the present results.
In light of our findings, we suggest postoperatively assessing CTC levels in early-stage NSCLC cases without chemotherapy indications. The effect of chemotherapy on recurrence, which is quite common even in early stages, should also be investigated over a possible threshold value.
The main limitation of our study is its retrospective design. The fact that it was a retrospective study brings with it the problem of obtaining follow-up data for the patients. Also, despite our diligent observance of oncological principles during wedge resection, additional manipulations may have occurred during processes such as pressing the parenchyma with a stapler. Finally, our inclusion criteria inevitably narrowed the study population to 98 cases.
In conclusion, although NTIT with wedge resection is theoretically well grounded, it is not clinically significant based on our findings, and its necessity should be tested in studies with larger case series that include the presence of CTCs prospectively. The technique may prove beneficial in re-evaluating chemotherapy indications through CTC detection, especially for patients in early stages who have not received chemotherapy.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Ethics approval
Local ethical committee approval of Ankara Keçiören Training and Research Hospital Ethical Committee (2012-KAEK-15/2399) was obtained for the study.
Authors’ contributions
MÇ,IT: Conceptualized and designed the study, co-wrote the paper; ESG: collected and analyzed data; GF: contributed to the organization and planning of the study; KA,SŞEG,PB: revised the final version of the manuscript.
Reference
1) Refaely Y, Sadetzki S, Chetrit A, Simansky DA, Paley M, Modan Bet al. A. The sequence of vessel interruption during lobectomy for non-small cell lung cancer: is it indeed important? J Thorac Cardiovasc Surg 2003; 125: 1313-20.
2) Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE et al. International Association for the Study of Lung Cancer Staging and Prognositic Factors Committee, Advisory Broads, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions: The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage grouping in the forthcoming (eight) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 39-51.
3) Hashimoto M, Tanaka F, Yoneda K, Takuwa T, Matsumoto S, Okumura Y et al. Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact Cardiovasc Thorac Surg 2014; 18: 775-83.
4) Duan X, Zhu Y, Cui Y, Yang Z, Zhou S, Han Yet al. Circulating tumor cells in the pulmonary vein increase significantly after lobectomy: A prospective observational study. Thorac Cancer 2019; 10: 163-9.
5) Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, GreystokeA et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011; 29: 1556-63.
6) Yasukawa M, Sawabata N, Kawaguchi T, Taniguchi S. Effectiveness of Intraoperative Pulmonary Wedge Resection of Tumor Site Before Lobectomy for Early Lung Adenocarcinoma. Anticancer Res 2019; 39: 6829-34.
7) Yasukawa M, Sawabata N, Kawaguchi T, Taniguchi S. Wedge Resection of Tumor Before Lobectomy for Lung Cancer Could Be a No-touch Isolation Technique. In Vivo 2020; 34: 779-85.
8) Sales JP, Wind P, Douard R, Cugnenc PH,Loric S. Blood dissemination of colonic epithelial cells during no-touch surgery for rectosigmoid cancer. Lancet 1999; 354: 392.
9) Steinert G, Schölch S, Niemietz T, Iwata N, García SA, Behrens B et al. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res 2014; 74: 1694-704.
10) Punnoose EA, Atwal S, Liu W, Raja R, Fine BM, Hughes BG et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 2012; 18:2391-401.
11) Hofman V, Bonnetaud C, Ilie MI,Vielh P, Vignaud JM, Fléjou JF et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res 2011; 17:827-35.
12) Lindsay CR, Blackhall FH, Carmel A, Fernandez-Gutierrez F, Gazzaniga P et al. EPAC-lung: pooled analysis of circulating tumour cells in advanced non-small cell lung cancer. Eur J Cancer 2019; 117: 60-8.
13) Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, GreystokeA et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011; 29: 1556-63.
14) Vasseur A, Kiavue N, Bidard FC, Pierga JY, Cabel L. Clinical utility of circulating tumor cells: an update. Mol Oncol 2021; 15: 1647-66.
15) Gray ES, Rizos H, Reid AL, Boyd SC, Pereira MR, Lo J et al. Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget 2015; 6: 42008-18.
16) Gadgeel SM. Role of Chemotherapy and Targeted Therapy in Early-Stage Non-Small Cell Lung Cancer. Am Soc Clin Oncol Educ Book 2017; 37: 630-9.
17) Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc 2019; 94: 1623-40.
18) Pathak R, Goldberg SB, Canavan M, Herrin J, Hoag JR, Salazar MC et al. Association of Survival With Adjuvant Chemotherapy Among Patients With Early-Stage Non-Small Cell Lung Cancer With vs Without High-Risk Clinicopathologic Features. JAMA Oncol 2020; 6: 1741-50.
19) National Comprehensive Cancer Network Non-small cell lung cancer. Version 3.2020. 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
20) Cheng F, Su L, Qian C. Circulating tumor DNA: a promising biomarker in the liquid biopsy of cancer. Oncotarget 2016; 7: 48832-41.
21) Hofman V, Ilie MI, Long E,Selva E, Bonnetaud C, Molina T et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch Assay™ and the isolation by size of epithelial tumor cell method. Int J Cancer 2011; 129:1651-60.
22) Bayarri-Lara C, Ortega FG, CuetoLadrón de Guevara A, Puche JL, Ruiz Zafra J, de Miguel-Pérez D et al. Circulating Tumor Cells Identify Early Recurrence in Patients with Non-Small Cell Lung Cancer Undergoing Radical Resection. PLoS One 2016; 11: e0148659.
23) Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M et al. Circulating mutant DNA to assess tumor dynamics. Nat Med 2008; 14: 985-90.
24) Hoffmann AC, Warnecke-Eberz U, Luebke T, Prenzel K, Metzger R, Heitmann M et al. Survivin mRNA in peripheral blood is frequently detected and significantly decreased following resection of gastrointestinal cancers. J Surg Oncol 2007; 95: 51-4.
25) Yumoto K, Eber MR, Berry JE, Taichman RS, Shiozawa Y. Molecular pathways: niches in metastatic dormancy. Clin Cancer Res 2014; 20: 3384-9.
26) Solomon J, Raškova M, Rösel D, Brábek J, Gil-Henn H. Are We Ready for Migrastatics? Cells 2021; 10: 1845.
27) Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med 1999; 5: 677-85.
28) Pittet MJ, Speiser DE, Liénard D, Valmori D, Guillaume P, Dutoit V et al. Expansion and functional maturation of human tumor antigen-specific CD8+ T cells after vaccination with antigenic peptide. Clin Cancer Res 2001; 7: 796s-803s.






