Summary
Chronic obstructive airway disease (COPD) is a progressive, irreversible and debilitating disease causing lung hyperinflation. Apart from smoking cessation and conventional medical treatment, lung volume reduction surgery (LVRS) has been used for several years but it involves a major thoracic surgery with high incidence of postoperative complications. In the past decade, different approaches of minimally invasive endoscopic lung volume reduction (ELVR) have been developed which differ in indication, mechanism of action, reversibility and are divided into two groups: blocking and non-blocking devices.The endobronchial valves belong to the group of blocking devices available and have the largest series of treated patients. These one way valves are used to occlude the most emphysematous and hence destroyed lobe of the lung. Two different types of valves are available on the market: endobronchial valves (EBV, Zephyr valves) and intrabronchial valves (IBV, Spiration valves). They differ in shape but have a similar mechanism of action.
In order to improve the outcome of the ELVR using valves, dedicated screening involving pulmonary function and exercise capacity testing as well as qualitative and quantitative CT analysis and perfusion scan are necessary. Numerous studies in the past years have shown the efficacy and complications following valve therapy. It has been demonstrated that patients with complete fissures show a more pronounced benefit and a significant target lobe volume reduction. Furthermore, unilateral implantation aiming at obtaining complete lobar occlusion has been more effective than the bilateral incomplete treatment. Regarding possible complications, apart from pneumothorax, COPD exacerbations, hemoptysis and valve migrations have been reported.
Summarizing, in comparison to LVRS, ELVR using valves is a less invasive alternative with the opportunity to improve shortness of breath, exercise capacity, and quality of life in the patients, who have reached the end of their conventional treatment options.
Introduction
Chronic obstructive airway disease (COPD) is a major cause of mortality and morbidity worldwide, currently constituting the fourth leading cause of death and it is estimated that it will occupy the 3rd place by 2020 [1]. Emphysema is present in approximately 1.8% of the population. It is a progressive, irreversible and debilitating disease, characterized by destruction of lung tissue as a result of inflammation caused by exposure to noxious inhaled agents for extended time. The most common cause of emphysema is cigarette smoking, but genetic, occupational, and environmental causes also account for up to 10% of cases [2]. Despite extensive public health initiatives aimed at discouraging cigarette smoking, smoking-related lung diseases remain a significant cause of disability and death worldwide.The alveolar destruction caused by these agents leads to impairment in gas exchange and elastic recoil of the lung causing thus air trapping with an increase in residual volume and hyperinflation which subsequently worsen the patients´ dyspnea perception. Because of the hyperinflation the respiratory muscles are forced to function at a mechanical disadvantage leading to decreased compliance of the chest wall and increase in work of breathing. As a consequence, patients experience chronic shortness of breath and limited exercise capacity and have thus a gradually declining quality of life. Smoking cessation and conventional medical treatment with inhaled bronchodilators (anticholinergics, beta-adrenergic agonists), anti-inflammatory medications (corticosteroids) and oral phosphodiesterase inhibitors constitute the mainstay of pharmacological treatment of COPD but are generally of limited benefit [3]. Long term oxygen therapy is recommended in patients with chronic respiratory failure and ventilatory support is indicated in patients with significant hypercapnia and related clinical symptoms. Finally, pulmonary rehabilitation and regular vaccination also play a very important role in the complex treatment strategy of COPD.
Surgical treatment for severe emphysema
Lung volume reduction surgery (LVRS) has been used for several years in order to reduce the size and the hyperinflation of the lung. This procedure was first described by Brantigan back in the fifties [4]. Damaged areas of lung are resected either via median sternotomy or via a less invasive video-assisted thoracoscopy in order to reduce the lung hyperinflation and in this way increase the breathing capacity. The benefits of LVRS were reported and confirmed in the National Emphysema Treatment Trial (NETT), which was a randomized controlled trial performed at 17 centers across the United States, comparing LVRS with optimal medical treatment [5]. 1218 patients were randomized and the selected subgroup of patients who were randomized to LVRS showed significant improvements in lung function, exercise capacity and quality of life. LVRS involves though a major thoracic surgery in a generally elderly population with limited breathing capacities that very frequently have clinically significant comorbidities. The NETT trial revealed a high incidence of postoperative complications; high incidence of serious cardiopulmonary complications, prolonged air leak and a three-month mortality rate of 5-10% were reported. Thus, despite its potential benefits fewer than 300 cases of LVRS are performed annually in the US and there are similarly small numbers in Europe [6].
Another surgical treatment for severe emphysema is lung transplantation. Accounting for 40% of all adult lung transplantations performed worldwide, end-stage COPD is the most common indication for lung transplantation [7]. Although single lung transplantation was in the past the predominant surgical therapy [8], the proportion of patients undergoing bilateral transplantation for COPD has increased significantly in the past decade. A total of 3,640 adult lung transplantations were reported in 2011 to the International Society for Heart and Lung Transplantation Registry [7]. Lung transplantation remains a major operative treatment available though to a limited number of patients, due to limited organ availability and access to specialized tertiary care centers.
In the past decade, different approaches for endoscopic lung volume reduction (ELVR) have been developed with the aim of reducing lung hyperinflation yet at the same time avoiding the morbidity, mortality and costs connected with LVRS as well as widening the indications to patients with severe comorbidities. These techniques differ in indication, mechanism of action, reversibility, as well as complications and are divided into two groups: blocking and non-blocking devices. All have been approved in Europe, but not by the US Food and Drug Administration to date. The aim of this review is to analyse the most widely studied devices for the ELVR, the endobronchial and intrabronchial valves.
Endoscopic lung volume reduction using valves
The endobronchial valves belong to the group of blocking devices available and have the largest series of treated patients. The characteristic feature of the endobronchial valves is the ability to block the entrance of air during inspiration, while permitting the emission of air and secretions during expiration. These one way valves are used to occlude the most emphysematous and hence destroyed lobe of the lung. With every expiration the amount of air in the treated lobe is reduced and this leads to volume reduction of the treated lobe reducing the overall lung volume and ideally inducing a complete lobar atelectasis, Figure 1.
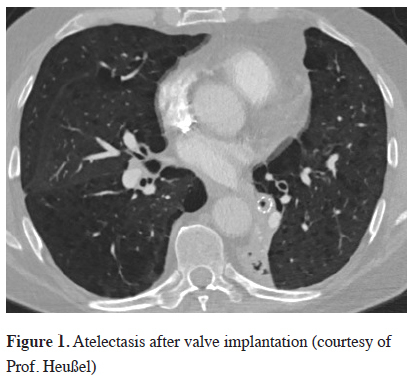 Click Here to Zoom |
Figure 1: Atelectasis after valve implantation (courtesy of Prof. Heußel) |
Two different types of valves are available on the market: endobronchial valves (EBV, Zephyr valves) and intrabronchial valves (IBV, Spiration valves). They differ in shape but have a similar mechanism of action; both devices are self-expanding and retained into a catheter that can be introduced through the working channel of a flexible bronchoscope. The choice between one of the two types is influenced more by the bronchial anatomy rather than by different outcomes after valve placement. Although no comparative trial has been published, the effect of both types of valves seems to be similar.
Patient selection criteria for valve therapy
Pulmonary function and exercise capacity testing
In order to improve the outcome of the ELVR using valves, dedicated screening, and selection of potential candidates is necessary. Not every patient with advanced COPD and severe emphysema is in principle suitable for a valve implantation. Expert opinion suggests that patients with COPD GOLD D (III-IV) may be considered. The patient selection criteria for most studies concerning the endobronchial valves have initially been adopted from the criteria previously used in the NETT trial [5]. Therefore, patients with an FEV1 of <45% predicted and both an RV of >150% predicted and a total lung capacity of >100% were initially enrolled. All criteria mentioned here are based on expert opinion.
Patients with an FEV1 of <40% predicted and an RV of >200% tend to benefit more from valves, although this reflects the clinical experience rather than published data. A DLCO of < 20% predicted is not a strict contraindication for bronchial valve placement. The lower the DLCO the greater the emphysematous destruction of the lungs, however a significant number of patients are unable to perform a DLCO assessment correctly because they are not able to breath-hold for 10 seconds. Patients should have stopped smoking at least 4 months prior to any intervention and have received optimal conventional medical treatment with inhaled bronchodilators and anti-inflammatory medications. Recurrent exacerbations/pneumonias with hospitalisations may exclude patients from ELVR. A distance in 6-minute walking test (6-MWT) of >140 m represents a parameter for better endurance and for the functional reserve of the patient. Patients should optimally have taken part in a pulmonary rehabilitation program. There is however till now no evidence if pulmonary rehabilitation should be performed prior to or after ELVR to achieve the best outcome. Both the 6-MWT and the requirement of pulmonary rehabilitation are surgical inclusion criteria to minimize the peri- and postoperative morbidity, and their relevance in the less invasive ELVR is still unclear.
Qualitative, Quantitative CT analysis and perfusion scan
The characterisation and quantification of emphysema is necessary for treatment planning and for monitoring of results. All patients require, in addition to the pulmonary function testing, a high resolution computer tomography (HRCT) prior to the intervention. Normally used in a low-dose protocol with a slice thickness of ≤1 mm, the HRCT enables the detection and quantification of the destruction of the peripheral lung tissue. Emphysema is divided into heterogeneous and homogeneous.
Depending on the emphysema predominance in patients with heterogeneous distribution, valve placement is possible in the upper lobes as well as in the lower lobes. In VENT and Euro-VENT the outcome was similar for both, without an increased risk for either of the group [9-10]. Homogeneous distribution of emphysema has not been extensively studied so far. A small case series showed that even patients with a homogeneous distribution could benefit [11]. Currently, patients with severe homogeneous emphysema are being recruited into a European Multicentre trial (IMPACT NCT02025205) to evaluate the effect of EBV placement in this patient subgroup.
Automated software programs help to visualize the severity and distribution of emphysema using the raw data and a threshold of -910 to -950 Hounsfield units (HU) and the emphysema can in this way be quantified automatically [12], Figure 2. Furthermore, new programs can determine additional parameters such as the heterogeneity index, the peripheral pulmonary vessel volume, low attenuation clusters (LACs) and also perform a three-dimensional analysis of the fissures, which have been shown to also play a role in predicting outcomes [13].
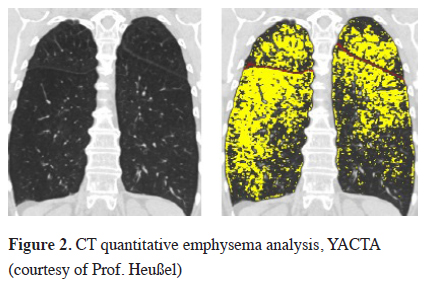 Click Here to Zoom |
Figure 2: CT quantitative emphysema analysis, YACTA (courtesy of Prof. Heußel) |
In lung parenchyma which has been destroyed by the emphysema, the perfusion is reduced. A perfusion scan can therefore be useful to confirm the target zone for ELVR and to verify the heterogeneity.
Collateral ventilation and fissure analysis
Collateral ventilation is an important predictor for the success of endobronchial valve placement. It has been defined as "the ventilation of alveolar structures through passages that bypass the normal airways" [14]. A post hoc subgroup analysis the VENT study demonstrated that patients with a complete interlobar fissure in the HRCT experienced very good outcomes following valve implantation [9]. A complete fissure is currently defined as being >90% complete between the target and adjacent lobes in at least one axis on the CT. A complete fissure seems to be a surrogate indicator for low interlobar collateral ventilation (CV). Detection of large parenchymal connections is associated with a significant air exchange independent of the central airways. Therefore, for patients with an incomplete fissure it is very unlikely for atelectasis of the treated lobe to occur as air can still enter the lobe via "the back door" even if central lobar occlusion is achieved. These patients will most probably not benefit from valve treatment. This finding was confirmed by the results of the Euro-VENT [10].
Besides CT based fissure analysis, CV can be quantified by an invasive catheter-based measurement using the Chartis® Pulmonary Assessment System (Pulmonx Inc., Redwood, Calif., USA). It uses an endobronchial catheter with a compliant balloon at the distal tip to block the airway that is to be treated with endobronchial valves. Air flows from the target lobe through the Chartis catheter and the amount of air flow as well as pressure generated beyond the tip can be visualized via the Chartis console and thus CV can be quantified [15]. Patients with no significant interlobar ventilation are the patients who will benefit from valve implantation and are classified as "CV negative"; patients with a high interlobar flow are classified as "CV positive" and these patients are not candidates for ELVR with valve implantation. In a prospective multicenter trial published in 2013 the accuracy of CV assessment using the Chartis® System was evaluated [16]. Eighty patients were evaluated and the results demonstrated that Chartis measurement can predict with 75% accuracy which patients will benefit significantly from ELVR with valves.
Zephir valve
The Zephyr® Valves (EBV, Pulmonx, Inc., Palo Alto, California, USA) have been studied most extensively. They are made of a nitinol mesh covered by silicon, with a double silicon membrane inside that opens during expiration and closes during inspiration, Figure 3. The valve is currently available in 3 different sizes. Measurement of the airway diameter in order to determine the appropriate size is required and it is performed with the introduction catheter. The catheter is advanced via the working channel of a flexible bronchoscope and is used to assess the size of the airway as well as deliver the appropriate size of valve. The first pilot study with this valve was published by Toma et al. in 2003 [17].
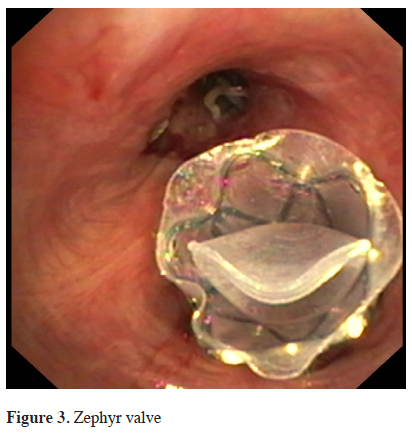 Click Here to Zoom |
Figure 3: Zephyr valve |
In the first and largest up to date randomized controlled trial, the Endobronchial Valve for Emphysema Palliation Trial (VENT), in patients with severe heterogeneous emphysema, endoscopic lung volume reduction with EBV was compared to best medical therapy [9]. In this trial 321 patients were randomized; 220 patients with advanced emphysema were treated by a complete occlusion of the targeted lobe (upper or lower lobe) by EBV and were compared to 101 patients who received only standard medical therapy. Primary endpoints of this study were safety and efficacy and the co-primary endpoints were percent change in FEV1 and 6-MWT. The difference between the 2 groups at 6-month follow-up was FEV1 increase in the EBV group by 6.8% (P = 0.002) and an improvement in 6-MWT by 5.8% (increase by 9.3 m). The most common adverse events within 90 days were COPD exacerbations (7.9%), pneumothorax (4.2%) and hemoptysis (5.6%). Although the mean between-group difference was significant, the results were not clinically relevant.
Some patients were "better responders" though, meaning that they had clinically significant changes in their clinical outcome measures. The subgroup analysis of patients with complete fissures though, showed a more pronounced increase in FEV1 and 6-MWT (FEV1 by 16.2% and 6-MWT by 7.7% at 6 months) and a significant target lobe volume reduction, whereas the patients with incomplete fissures showed only minimal difference to the control group. Fissure completeness as assessed on HRCT scan was analysed individually as a marker of collateral ventilation and was shown to be an independent predictor of response to treatment. Hence a complete occlusion of the treated lobe with absence of collateral ventilation proved to be prognostic factors for significant improvement in FEV1 and 6-MWT. In the recently published European cohort of the VENT study, patients in whom target lobe volume reduction >50% (TLVR) was achieved had much greater improvements in clinical outcomes compared to a TLVR <20%. Therefore, real volume reduction seems to be also an important point for a better outcome. Complication rates such as COPD exacerbations were the same in all groups [18].
Regarding the longer term effects of Zephyr valves implantation, a study by Venuta et al. [19] assessed the long term effects and also the clinical outcomes over 5 years. 40 patients were treated with Zephyr valves prior to Chartis being available. FEV1 improved from 0.88 ± 0.3l to 1.1 ± 0.2l after 3 months and this change was sustained on follow-up after 3 and 5 years. RV decreased by a mean of 500 ml and both 6-MWT and mMRC score improved significantly. Most of the improvements were seen in the first year, but they were sustained over a prolonged period although the follow up was constrained to only 9 out of the 40 patients for the full 5 years. No severe complications due to the valves were observed. There were deaths reported during the long follow-up patients, but none of the deaths were associated with valve placement. In view of the advanced disease the mean and median survival was 36 ± 4.3 months and 30 ± 4.6 months respectively. Survival was noted at 1, 3 and 5 years to be 81.6%, 47.4% and 22.4% respectively.
After the recognition of possible predictive factors more randomized controlled studies were carried out in which only patients with low collateral ventilation, were treated by complete occlusion of one lobe. In the so-called Believer HiFi study [20], 25 patients with complete fissures, were treated by valve placement and compared with 25 patients of the control group. This study did not take into account the results of the Chartis® measurement that was performed before valve implantation though. Three months after the valve therapy FEV1 in the treatment group increased by a median of 8.77% (IQR 2.27-35.85) versus 2.88% (0-8.51) in the control group (Mann-Whitney P = 0.0326). Significant results have also been seen in the 6-MWT.
Another study from last year compared the clinical outcomes in upper versus lower lobe EBV treatment in severe emphysema [21]. Of the 331 patients treated, 60 had low interlobar collateral ventilation and successful lobar exclusion (45 patients with upper lobe treatment and 15 patients with lower lobe treatment). A higher destruction score (70.3 vs. 60.7%; P = 0.0010) and a higher heterogeneity index (24 vs. 13%; P = 0.0005) for the upper lobe cohort were the only differences in the baseline characteristics between the two groups. At 180 days, both groups had improved clinically. There were no significant differences in mean changes or responder rates of FEV1 (+23.8 vs. +22.9%), the SGRQ score (-6.50 vs. -7.53 points), 6-MWT (+24.1 vs. +44.0 m), target lobe volume reduction (-1,199 vs. -1,042 ml), or in the adverse event rate between both cohorts. This study showed that patients with lower and upper lobe predominant emphysema benefit equally from EBV therapy when interlobar collateral ventilation is low and lobar exclusion is achieved.
Finally, in the recently published STELVIO study [22], a total of 68 patients were randomized to a treatment arm and a control arm. In all 34 patients who received valve treatment, significant collateral ventilation had been excluded previously by Chartis® measurement. Six months after valve implantation, a significant difference in FEV1 (161 ml vs. 21 ml), forced vital capacity (VC) (416 ml vs. 69 ml) and the 6-MWT (60 m vs. -14 m) have been seen between the two groups in favor of the treatment arm. Furthermore, significant differences in the SGRQ score, as well as the lobar volume reduction have been reported. This study has thus further confirmed the latest conducted randomized clinical trials and has shown that valve therapy after appropriate patient selection is an effective treatment option for patients with advanced emphysema.
Spiration valve
The intrabronchial valve (IBV, Spiration®, Olympus, Tokyo, Japan) consists of a polymer covered framework made out of nitinol (a nickel and titanium alloy) in the shape of an umbrella, Figure 4. The valve is secured to the bronchial wall by 5 hook-like anchors and can be removed by grasping and pulling on its proximal central rod with forceps. 4 different sizes (5, 6, 7 and 9 mm) are currently available. Three multicenter studies in the past decade enrolled patients with upper lobe predominant emphysema. At the beginning valves were placed unilaterally in the upper lobes and were initially well tolerated by patients without any serious complications. The most frequent adverse events recorded were pneumonia, COPD exacerbation, dyspnea, hemoptysis, chest pain and pneumothorax [23-25].
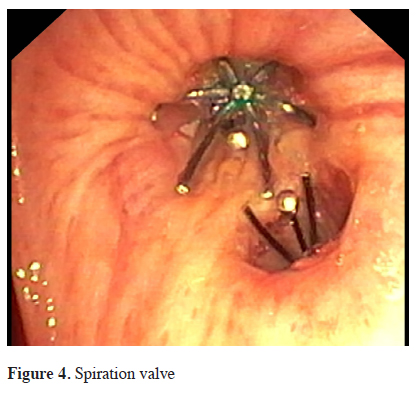 Click Here to Zoom |
Figure 4: Spiration valve |
It is interesting to note that in the pilot study by Stermann et al. 12.1% of the patients sustained a pneumothorax [25]. Following the observation of a higher incidence of pneumothorax occurring with complete lobar occlusion (especially of the left upper lobe), the investigators modified their strategy during the study and continued with bilateral treatment keeping open the lingula and avoiding complete lobar occlusion. The aim of this treatment strategy was not the actual lung volume reduction but rather a redirection of air to less destroyed and better perfused lung tissue, improving thus the ventilation/perfusion match. The results of this trial showed no modification of functional parameters (FEV1, total lung volume and exercise tests were unchanged) but a significant improvement of quality of life (SGRQ = -8.2 points at 6 months). All three abovementioned studies showed a significant improvement in quality of life questionnaire, but they could not demonstrate a statistically significant improvement of lung function parameters and 6-MWT.
In a multicenter, blinded, sham-controlled study by Ninane et al. [26], 73 patients were included. All patients had upper lobe predominant severe emphysema, 37 of them were randomized to bronchoscopy with implantation of IBV valves and 36 had a sham bronchoscopy. The treatment consisted of bilateral upper lobe valve implantation with incomplete occlusion of the target lobe. Overall the procedure and devices were well tolerated and there were no differences in adverse events between both groups. The most frequent complications were pneumonia (3.6-4.2%), pneumothorax (4.2-4.5%), hemoptysis (5.4-6.1%) and exacerbation of COPD (7.9%). At 3 months follow-up, there was no significant difference in lung function, health-related
quality of life (assessed by SGRQ) or breathlessness. Treatment with IBV without complete occlusion was safe but ineffective and no functional improvement was observed. The improvement in SGRQ in both groups was attributed to a significant placebo effect. Another randomized, sham procedure controlled, double-blind multicenter trial from Wood et al. [27], 277 subjects were enrolled at 36 centers. The primary effectiveness measure was a significant improvement in disease-related quality of life (St. George"s Respiratory Questionnaire) and changes in lobar lung volumes. The primary safety measure was a comparison of serious adverse events. There were 6/121 (5.0%) responders in the treatment group at 6 months, significantly >1/134 (0.7%) in the control group [Bayesian credible intervals (BCI), 0.05%, 9.21%]. Lobar volume changes were significantly different with an average decrease in the treated lobes of -224mL compared with -17mL for the control group (BCI, -272, -143). The proportion of responders in St. George"s Respiratory Questionnaire was not greater in the treatment group. There were significantly more subjects with a serious adverse event in the treatment group (n = 20 or 14.1%) compared with the control group (n = 5 or 3.7%) (BCI, 4.0, 17.1), but most were neither procedure nor device related.
Eberhardt et al. demonstrated that a unilateral procedure with the aim of a complete lobar occlusion is more effective than bilateral incomplete treatment [28]. Twenty two patients were enrolled and randomised in this study, 11 patients received unilateral IBV valve implantation and 11 patients bilateral upper-lobe implantation. The aim of the unilateral valve placement was a total occlusion of one lobe, the bilateral treatment aimed at an incomplete closure. The 30 and 90 day follow-up showed a significant difference in lung function (FEV1 = +21.4 vs. –0.03%) and 6-MWT, as well as in mMRC and SGRQ scores in the unilateral treated group. One patient suffered a pneumothorax in the unilateral group, and two patients in the bilateral group needed treatment for a respiratory event. These results demonstrated that the unilateral procedure aimed at obtaining complete lobar occlusion is more effective than the bilateral incomplete treatment and that, given the superior outcome, the increased risk of pneumothorax may be acceptable.
Complications of valve therapy
Although endoscopic valve therapy is a minimally invasive procedure, it is associated with a complication risk. Within the first 3 months after valve implantation in the VENT study COPD exacerbations, hemoptysis, pneumothoraxes, and also valve migrations were reported [9]. In particular, pneumothorax has been of high importance, since in the recent years a significant increase in its incidence has been observed, Figure 5. While in the VENT study a pneumothorax rate of only 4.2% was recorded, in the follow-up studies the pneumothorax rates were between 8-25% [20,29]. The cause of this increasing incidence is the optimized patient selection: a complete fissure seems not only to be a predictor of a successful valve therapy, but also a predictor of pneumothorax occurrence. A very rapid volume displacement by an atelectasis of the treated lobe can lead to a tear in the expanding untreated collateral lobe and thus to a postinterventional pneumothorax. Thus with complete fissures being a predictor of successful treatment as well as of pneumothorax it is likely that patients who suffer from pneumothorax after valve implantation will also benefit from valve treatment. A retrospective study showed that patients with a pneumothorax after valve therapy experienced a lobar volume reduction of 65% [30]. In another study it could be demonstrated that clinical success will occur only in patients who in the course after the successful pneumothorax management also develop an atelectasis [31]. Nevertheless, pneumothorax is a serious complication that usually requires intrapleural drainage and in about half the cases requires also the removal of at last one valve. An algorithm should be developed in order to manage these pneumothoraces that occur after valve placement. Chest tube insertion is the first step but if this fails to re-expand the lung, valve removal or video thoracoscopy to seal the leak may be the next step. Because the pneumothorax occurs in 76% of cases within the first three days after valve implantation, a stationary monitoring 48-72 hours is advised [32].
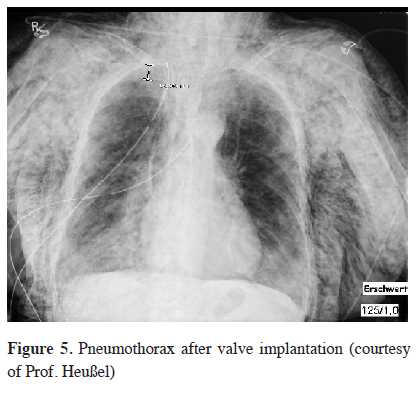 Click Here to Zoom |
Figure 5: Pneumothorax after valve implantation (courtesy of Prof. Heußel) |
A 48-hour postinterventional bed-rest seems to minimize the risk of pneumothorax [29]. In this recent study von Herzog et.al, 72 consecutive COPD patients with severe homogeneous or heterogeneous emphysema and negative collateral ventilation status assessed by the Chartis console, were treated with EBV. 32 patients were treated with standard medical care (SMC) without restriction to bed rest and 40 patients followed a modified medical care (MMC) that included 48 hours strict bed-rest and, if needed, 16 mg codeine up for cough to three times a day (TID). The frequencies of pneumothorax were compared between the two groups. In the 48-hour bed-rest group a significant lower rate of pneumothorax was observed (P = 0.02) and no significantly increased incidence of thromboembolic events, infections or complications. However, the number of patients of this study is too small, so further studies to verify protective factors of pneumothorax are required.
As a conclusion emphysema is a debilitating lung disease and a global health burden. Endoscopic valve implantation is a new treatment option for patients with advanced COPD and severe emphysema. Nevertheless, medical therapy, pulmonary rehabilitation, as well as smoking cessation remain the basis of therapy. In comparison to LVRS, the endoscopic procedures are less invasive alternatives with the opportunity to improve shortness of breath, exercise capacity, and quality of life in the patients, who have reached the end of their conventional treatment options.
The careful patient selection which should include assessment of collateral ventilation, emphysema heterogeneity and distribution, the amount of hyperinflation and comorbidities are all factors that need to be carefully considered before treatment. Patients who are potential candidates, according to experts in the field, should have in the lung function testing ideally FEV1<40% and RV>200%. Even though patients with FEV1 of under 20% were excluded from older trials since they were considered a high risk group, it has been shown in clinical practice that these patients also benefit from these interventional techniques.
There is increasing evidence that endobronchial valves are particularly effective in patients with heterogeneous emphysema, where total lobar exclusion is achieved and current data point even to a survival advantage for those patients with lobar atelectasis. The best clinical and functional results seem to be correlated with the development of atelectasis. Without atelectasis the improvement is generally modest or absent.
The development of atelectasis or rapid volume reduction is associated with a risk of pneumothorax as a consequence of the nontreated ipsilateral lobe expanding quickly and the visceral pleura tearing. The pneumothorax usually occurs within 24-72 hours after the procedure and may require an intercostal chest tube. New research evidence seems to suggest that patients who develop a pneumothorax after EBV placement showed greater improvement in FEV1 and target lobe volume reduction. Therefore, this risk may be acceptable for the patient and the bronchoscopist in view of the potential clinical benefit. Finally, the main advantage of both valves is their removability and safety. Currently however there is no comparative study available addressing the question of superiority of one valve versus the other.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The author received no financial support for the research and/or authorship of this article.
Reference
1) Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. Available from: http://www.goldcopd.org/
2) Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006; 28: 523-32.
3) Ambrosino N, Simonds A. The clinical management in extremely severe COPD. Respir Med 2007; 101: 1613-24.
4) Brantigan OC, Mueller E. Surgical treatment of pulmonary emphysema. Am Surg 1957; 23: 789-804.
5) Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348: 2059-73.
6) Chang AC, Chan KM, Martinez FJ. Lessons from the national emphysema treatment trial. Semin Thorac Cardiovasc Surg 2007; 19: 172-180.
7) Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report-2013; focus theme: age. J Heart Lung Transplant 2013; 32: 965-78.
8) Trulock EP, Christie JD, Edwards LB, Boucek MM, Aurora P, Taylor DO, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report – 2007. J Heart Lung Transplant 2007; 26: 782-95.
9) Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010; 363: 1233-44.
10) Herth FJ, Noppen M, Valipour A, Leroy S, Vergnon JM, Ficker JH, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J 2012; 39: 1334-42.
11) Eberhardt R, Heussel CP, Kreuter M, Weinheimer O, Herth FJ. Bronchoscopic lung volume reduction in patients with severe homogeneous lung emphysema: a pilot study. Dtsch Med Wochenschr 2009; 134: 506-10.
12) Heussel CP, Herth FJ, Kappes J, Hantusch R, Hartlieb S, Weinheimer O, et al. Fully automatic quantitative assessment of emphysema in computed tomography: comparison with pulmonary function testing and normal values. Eur Radiol 2009; 19: 2391-402.
13) Schuhmann M, Raffy P, Yin Y, Gompelmann D, Oguz I, Eberhardt R, et al. Computed tomography predictors of response to endobronchial valve lung reduction treatment. Comparison with chartis. Am J Respir Crit Care Med 2015; 191: 767-74.
14) Cetti EJ, Moore AJ, Geddes DM. Collateral ventilation. Thorax 2006; 61: 371-3.
15) Gompelmann D, Eberhardt R, Michaud G, Ernst A, Herth FJ. Predicting atelectasis by assessment of collateral ventilation prior to endobronchial lung volume reduction: a feasibility study. Respiration 2010; 80: 419-25.
16) Herth FJ, Eberhardt R, Gompelmann D, Ficker JH, Wagner M, Ek L, et al. Radiological and clinical outcomes of using Chartis to plan endobronchial valve treatment. Eur Respir J 2013; 41: 302-8.
17) Toma TP, Hopkinson NS, Hillier J, Hansell DM, Morgan C, Goldstraw PG, et al. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet 2003 15; 361: 931-3.
18) Valipour A, Herth FJ, Burghuber OC, Criner G, Vergnon JM, Goldin J, et al. Target lobe volume reduction and COPD outcome measures after endobronchial valve therapy. Eur Respir J 2014; 43: 387-96.
19) Venuta F, Anile M, Diso D, Carillo C, De Giacomo T, D"Andrilli A, et al. Long-term follow-up after bronchoscopic lung volume reduction in patients with emphysema. Eur Respir J 2012; 39: 1084-9.
20) Davey C, Zoumot Z, Jordan S, McNulty WH, Carr DH, Hind MD, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomized controlled trial. Lancet 2015; 386: 1066-73.
21) Eberhardt R, Herth FJ, Radhakrishnan S, Gompelmann D. Comparing Clinical Outcomes in Upper versus Lower Lobe Endobronchial Valve Treatment in Severe Emphysema. Respiration. 2015; 90: 314-20.
22) Klooster K, ten Hacken NH, Hartman JE, Kerstjens HA, van Rikxoort EM, Slebos DJ. Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation. N Engl J Med 2015; 373: 2325-35.
23) Wood DE, McKenna RJ, Jr., Yusen RD, Sterman DH, Ost DE, Springmeyer SC, et al. A multicenter trial of an intrabronchial valve for treatment of severe emphysema. J Thorac Cardiovasc Surg 2007; 133: 65-73.
24) Coxson HO, Nasute Fauerbach PV, Storness-Bliss C, Muller NL, Cogswell S, Dillard DH, et al. Computed tomography assessment of lung volume changes after bronchial valve treatment. Eur Respir J 2008; 32: 1443-50.
25) Sterman DH, Mehta AC, Wood DE, Mathur PN, McKenna RJ, Jr., Ost DE, et al. A multicenter pilot study of a bronchial valve for the treatment of severe emphysema. Respiration 2010; 79: 222-33.
26) Ninane V, Geltner C, Bezzi M, Foccoli P, Gottlieb J, Welte T, et al. Multicentre European study for the treatment of advanced emphysema with bronchial valves. Eur Respir J 2012; 39: 1319-25.
27) Wood DE, Nader DA, Springmeyer SC, Elstad MR, Coxson HO, Chan A, et al. The IBV Valve trial: a multicenter, randomized, double-blind trial of endobronchial therapy for severe emphysema. J Bronchology Interv Pulmonol. 2014; 21: 288-97.
28) Eberhardt R, Gompelmann D, Schuhmann M, Heussel CP, Herth FJ. Complete unilateral vs partial bilateral endoscopic lung volume reduction in patients with bilateral lung emphysema. Chest 2012; 142: 900-8.
29) Herzog D, Poellinger A, Doellinger F, Schuermann D, Temmesfeld-Wollbrueck B, Froeling V, et al. Modifying Post-Operative Medical Care after EBV Implant May Reduce Pneumothorax Incidence. PLoS One 2015; 10: e0128097.
30) Gompelmann D, Herth FJF, Slebos DJ, Valipour A, Ernst A, Criner GJ, et al. Pneumothorax following Endobronchial Valve Therapy and Its Impact on Clinical Outcomes in Severe Emphysema. Respiration 2014; 87:485-91.






