2Department of Thoracic Surgery, Faculty of Medicine, University of Erciyes, Kayseri, Turkey DOI : 10.26663/cts.2023.0010.
Summary
Pulmonary sequestration is defined as a segment of the lung that is supplied by one or more systemic arteries and has no connection with normal bronchial structures. This rare lung condition is basically classified into two different types: extralobar sequestration (ELS), which has its own pleura, and intralobar sequestration (ILS), which is in structural continuity with normal lung tissue. While aberrant arterial supply is usually derived from the thoracic aorta, abdominal aorta, celiac artery, splenic artery, and intercostal artery, it can also rarely be provided from the subclavian, left gastric, phrenic, and coronary arteries. This patient group can be asymptomatic but may present with complaints such as cough, fever, hemoptysis, and chest pain when symptomatic. Surgery is currently the treatment of choice for such patients.Here, we present a rare and fatal case of pulmonary sequestration supplied by the pulmonary artery in a neonate who was born at 32 weeks of gestation weighing 1980 g, required mechanical ventilatory support, and was operated on while intubated on the 32nd day of birth to provide curative treatment.
Introduction
Pulmonary sequestration (PS) is defined as a segment of the lung that is supplied by one or more systemic arteries and has no connection with normal bronchial structures. Although it was first described by Huber (in 1777), the term “sequestration” was first introduced in 1946 [1].Pulmonary sequestration is classified into intrapulmonary and extrapulmonary sequestrations depending on the presence of its own pleura. Although most of the sequestrations are supplied by the thoracic aorta and abdominal aorta alone, they can also receive blood supply from more than one abnormal systemic artery [2].
Both types of PS can be asymptomatic; however, the most common clinical symptoms include cough, sputum, fever, and hemoptysis when they are symptomatic [3].
Case Presentation
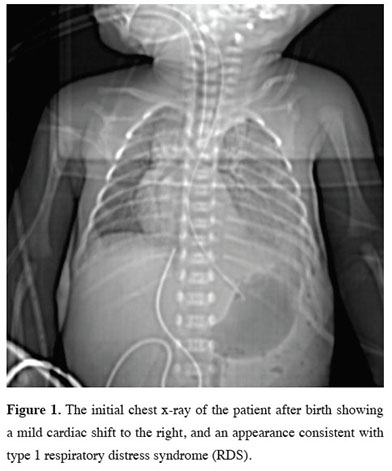
Our patient was born at 32 weeks of gestation weighing 1980 g from a 33-year-old mother who became pregnant through in vitro fertilization. The patient, who was non-invasively followed up with a pre-diagnosis of adenoid cystic malformation in the intrauterine period, was admitted to the neonatal intensive care unit and intubated due to severe dyspnea immediately after birth. His chest x-ray showed an appearance consistent with respiratory distress type 1 and a pulmonary mass (Figure 1).
 Click Here to Zoom |
Figure 1: The initial chest x-ray of the patient after birth showing a mild cardiac shift to the right, and an appearance consistent with type 1 respiratory distress syndrome (RDS). |
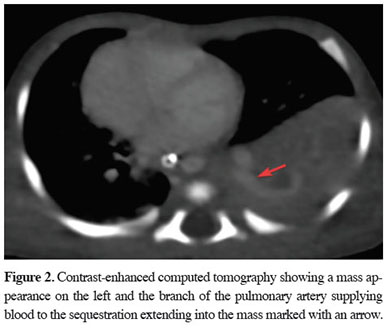
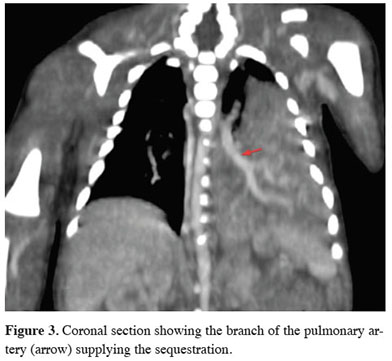
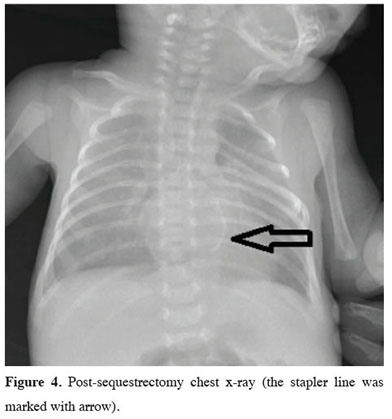
Surfactant therapy was administered to contribute to lung maturation. The patient with oxygen desaturation on mechanical ventilation was suspected to have pulmonary hypertension, therefore underwent a cardiac echo, which revealed PDA. Contrast-enhanced computed tomography of the chest (Figures 2,3) taken for the mass appearance was reported as a space-occupying formation of soft tissue density, extending from the left lung lower lobe superior segment to the basal segments, is notable. The soft tissue lesion appears to have no connection with the bronchial structure. The inferior branch of the left main pulmonary artery is markedly large and enters the soft tissue area in the lower posterior basal segment of the left lung. The clinically unstable patient with a persistent desaturation problem developed cardiopulmonary arrest once. He responded to cardiopulmonary resuscitation (CPR), achieving partial recovery in the clinic during the follow-up. After interviewing the patient’s family, a surgery decision was taken on the 32nd day of birth. The patient underwent a left thoracotomy. Exploration showed extrapulmonary sequestration with its own pleura on the left. It was easily isolated from the surrounding lung. It was observed that the sequestration artery originated from the pulmonary artery and the vein was drained into the pulmonary vein by extending along the same track. The artery and vein of the sequestration area that was found to have no connection with the bronchial structure were cut using a stapler, and sequestrectomy was completed. Mechanical ventilatory support was discontinued due to the dramatic improvement in respiratory parameters on the fourth day of the follow-up of the patient, who was again on mechanical ventilation postoperatively. The chest tube was removed on the sixth day of the follow-up. The patient was discharged in an extubated state on the 43rd day of hospitalization (Figure 4). Written informed consent was obtained from the parents for the publication of his data.
 Click Here to Zoom |
Figure 2: Contrast-enhanced computed tomography showing a mass appearance on the left and the branch of the pulmonary artery supplying blood to the sequestration extending into the mass marked with an arrow. |
 Click Here to Zoom |
Figure 3: Coronal section showing the branch of the pulmonary artery (arrow) supplying the sequestration. |
 Click Here to Zoom |
Figure 4: Post-sequestrectomy chest x-ray (the stapler line was marked with arrow). |
Discussion
Considering that some cases of PS are asymptomatic and may not be detected at all, it is not possible to state a precise incidence. However, post-mortem pediatric and antenatal studies have reported an incidence of 0.15-1.8% for PS [4].The most common site of involvement is the left lower lobe, followed by the right lower lobe. While PS is most commonly located in the abdomen other than the chest, it can also occur in different locations such as the neck [5,6].
Considering pediatric cases reported in the literature, approximately one-third of the patients are asymptomatic. The manifestations of symptomatic PS include respiratory tract infections [6].
Although large series of PS in the literature does not mention that the clinic of the patients may be severe enough to require respiratory and circulatory support [3,6], there are rare cases with such severe clinical manifestations presented in the form of a case report. Among these, there is a case report of PS supplied by the pulmonary artery in a premature infant with a severe clinic requiring early surgery, as in our patient [7,8,9].
Although chest x-ray is the first-line diagnostic test, contrast-enhanced computed tomography is sufficient for diagnosis today. This technique can also clearly demonstrate arterial supply. For this reason, pulmonary angiography has nowadays lost its former popularity. Apart from that, MRI and ultrasound are used as diagnostic techniques. However, diagnostic studies on both pediatric and adult patients have reported a quite high rate of misdiagnosis for these techniques [3,6].
Our patient was also followed up as having adenoid cystic malformation in the intrauterine period. The diagnosis could only be clarified by postnatal tomography.
One of the basic characteristic features of sequestration is systemic arterial supply. As in our patient, sequestration that receives blood supply from the pulmonary artery is very rare. As a matter of fact, a study of 2625 patients with PS in the literature identified arterial supply in 1808 patients, reporting pulmonary arterial supply in only 5 of them. Furthermore, a study of 208 pediatric patients found no pulmonary arterial supply at all [3,6].
As is understood from this information, pulmonary arterial supply is very rare in cases of PS. There is no comment in the literature about the relationship of this condition with the clinical conditions of the patients.
Surgery appears to be the most effective treatment approach currently. Apart from this, it is necessary to be cautious about congenital diaphragmatic hernia and other anomalies accompanying ELS, especially in ELS. When diagnosed, it may be appropriate to surgically treat these in the same session [6].
Another important point in surgery is that more than one arterial supply is seen in 20% of PS cases. Although a second arterial supply was not noted in our patient, it should be kept in mind that the arteries travel in the subpleural area. These are the main points to keep in mind to avoid a complication.
In fact, the debates on the surgery are two-dimensional. The first is whether to intervene in asymptomatic patients, while the other is about the timing of surgery.
The prevailing belief is that all patients should be referred to surgery after the diagnosis is confirmed. This approach seems reasonable to prevent future complications [3,6].
Nevertheless, there is no clear approach in the literature on timing. Infections have been shown to cause mortality and morbidity in children. While this frequency was the highest in the pediatric group in the first six months, it was found to be the lowest between 6 months and 1 year of age. Therefore, the recommended timing of surgery is between 6 months and 1 year of age [3].
As mentioned earlier in the literature, the diagnosis of our patient was challenging due to the rarity of pulmonary supply. The patient had a cardiac arrest while waiting for PDA closure and an appropriate time for surgery. Accordingly, the patient was operated on after discussing with the family, without discontinuation of ventilatory support. The patient achieved a dramatic improvement postoperatively. Accordingly, ventilation could be withdrawn, the chest tubes were removed, and finally, he was discharged.
Thoracotomy is the traditional approach adopted in surgery. However, it is of note that video-assisted thoracic surgery (VATS) has provided very good results in the pediatric and adult groups [6,10]. We preferred thoracotomy for our patient because of severe respiratory problems and low birth weight. However, VATS should not be excluded as a surgical treatment technique even for these patients.
In conclusion, it should be kept in mind that PS can be severe enough to cause severe respiratory distress in newborns, requiring emergency surgical intervention even though some of the cases are asymptomatic. Moreover, pulmonary arterial supply can be seen in sequestrations. We achieved curative results in the patient after surgery. Therefore, surgery should be performed as soon as possible after the clinical stabilization of the patients, albeit partially. It should be noted that surgery is the only known curative treatment for these patients.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support
Authors’ contributions
OFD,OO,OT: conceived and designed the current case report, co-wrote the paper, collected the clinical data. The authors discussed the case under the literature data together and constituted the final manuscript.
Reference
1) Pryce DM. Lower accessory pulmonary artery with intralobar sequestration of lung: a report of seven cases. J Pathol Bacteriol 1946; 58: 457-67.
2) Corbett HJ, Humphrey GME. Pulmonary sequestration. Paediatr Respir Rev 2004; 5: 59-68.
3) Wei Y, Li F. Pulmonary sequestration: a retrospective analysis of 2625 cases in China. Eur J Cardiothorac Surg 2011; 40: 39-42.
4) Savic B, Birtel FJ, Tholen W, Funke HD, Knoche R. Lung sequestration: report of seven cases and review of 540 published cases. Thorax 1979; 34: 96-101.
5) Clements BS, Warner JO. Pulmonary sequestration and related congenital bronchopulmonary vascular malformations: nomenclature and classification based on anatomical and embryological considerations. Thorax. 1987; 42: 401-8.
6) Zhang N, Zeng Q, Chen C, Yu J, Zhang X. Distribution, diagnosis, and treatment of pulmonary sequestration: Report of 208 cases. J Pediatr Surg 2019; 54: 1286-92.
7) Ojha S, Srivastava P, Poonia A, Bansal R. Thoracoscopic excision of double-pulmonary sequestration in left haemithorax, without anomalous blood supply (supply from pulmonary artery) in a neonate: First case. J Minim Access Surge 2021: 17; 556-8.
8) Rammos KS, Foroulis CN, Rammos CK, Andreou A. Prenatal interventional and postnatal surgical therapy of extralobar pulmonary sequestration. Interact Cardiovasc Thorac Surg 2010: 10; 634-5.






