2Department of Thoracic Surgery, School of Medicine, Ege University, İzmir, Turkey
3Department of Thoracic Surgery, Karşıyaka State Hospital, İzmir, Turkey DOI : 10.26663/cts.2016.0002
Summary
Background: The gold standard of treatment for non-small cell lung cancer (NSCLC) is anatomic pulmonary resection. Wider resection methods may be preferred due to the size or anatomic location of the tumor. One method of choice is bilobectomy due of the anatomy of the right lung.Materials and Methods: This study retrospectively analyzed 93 patients who were diagnosed with NSCLC and had bilobectomy, complete resection, and mediastinal lymph node dissection at our center between January 2005 and April 2013.
Results: Forty-seven patients underwent superior bilobectomy (sBL), and 46 patients underwent inferior bilobectomy (iBL). Bilobectomy was performed due to fissure invasion in 51 (58.1%) patients, internal or external bronchial tumor invasion in 31 (33.3%) patients, external bronchial lymph node invasion in six (6.4%) patients, and vascular invasion in two (2.2%) patients. The bronchial invasion-based indications were significantly higher in the iBL group compared to the fissure invasion-based indications in the sBL group (P < 0.001).
Conclusions: Bilobectomy leads to a substantial amount of parenchymal loss in the right lung, but it is a procedure that should be performed under the necessary conditions. It is obvious that performing bilobectomy under proper indications would result in good outcomes for lung cancer patients.
Introduction
The gold standard of treatment for non-small cell lung cancer (NSCLC) is anatomic pulmonary resection [1,2]. Wider resection methods may be preferred due to the size or anatomic location of the tumor. One method of choice because of the anatomy of the right lung is bilobectomy. Bilobectomy is the term used when the middle lobe lobectomy added upper or lower lobectomy in addition to the right and it was first performed by Churchill in 1933 [3]. Mid-upper lobectomy is called to bilobectomy superior or upper, mid-lower lobectomy is called to inferior or lower bilobectomy [2,3]. The present study analyzed patients who underwent bilobectomy due to non-small cell lung carcinoma at our center, and the outcome of the patients were investigated.Methods
This study retrospectively analyzed the patients who were diagnosed with non-small cell lung cancer and had bilobectomy, complete resection, and mediastinal lymph node dissection at our center between January 2005 and April 2013. We declare that the study was performed in accordance with the ethical standards laid down in the Helsinki Declaration of 1975, as revised in 1983. The patients who had a bilobectomy due to benign pathology and the patients who had undergone a sleeve resection were excluded from the study. Data was retrospectively collected from the patient files. The parameters analyzed included gender, age, bilobectomy type, neoadjuvant therapy, histopathological type, pathological stage, postoperative complications, mortality, reason for bilobectomy, and stump closure techniques (stapler vs primary). Pathological staging was done according to the seventh TNM staging system. In the presence of mediastinal lymphadenopathy, EBUS, mediastinoscopy and/or VATS were used for staging. All patients were intubated with a double-lumen intubation tube. Complete systematic mediastinal lymph node dissection was added to the bilobectomy procedure that was performed after posterolateral thoracotomy in all patients.
Statistical Methods
The data was analyzed using the Statistical Package for the Social Sciences (SPSS) 20 software. The quantitative data was analyzed using the Kolmogorov-Smirnov test for the compatibility with normal distribution, and parametric methods were used to analyze the variables with normal distribution and homogeneous variations, and non-parametric methods were used to analyze the variables without normal distribution and homogeneous variations. Two independent groups were compared using the independent t-test and the Mann-Whitney U-test. After the main factor was controlled for quantitative data, the correlations of the variables with each other were analyzed using the partial correlation test, and the categorical data was compared using Pearson"s chi-square test. The effects of the factors on mortality and lifetime were analyzed using the Kaplan-Meier method (product-limit method), and the effects of prognostic variables on lifetime according to the main factor were measures using the Cox regression analysis. Quantitative data were expressed in mean ± std. (standard deviation) and median ± IQR values in the tables. Categorical data were expressed in number (n) and percentage (%). The data were analyzed at the 95% confidence interval and P < 0.05 was considered significant.
Results
Among 93 patients, who had bilobectomy with the diagnosis of NSCLC, 81 (76%) were male and 12 (24%) were female, with a mean age of 60 ± 10.5 (range: 23-79 years). Forty-seven patients underwent superior bilobectomy (sBL), and 46 patients underwent inferior bilobectomy (iBL) (Table 1). Based on histopathological type, 57 (61.3%) patients were in the squamous cell carcinoma, 28 (30.1%) patients were in the adenocarcinoma, and eight (8.6%) patients were in the other NSCLC group. Patients" pathological stage were IA in 15, IB in 26, IIA in 15, IIB in 18 and III3A in 19 cases.Table 1: Demographics - patient distribution
The patients underwent bilobectomy due to four reasons under the categories of internal or external bronchial tumor invasion, fissure invasion, external bronchial metastatic lymph node invasion, and vascular invasion. Bilobectomy was performed due to fissure invasion in 54 (58.1%) patients, internal or external bronchial tumor invasion in 31 (33.3%) patients, external bronchial lymph node invasion in six (6.4%) patients, and vascular invasion in two (2.2%) patients. In the iBL group, bilobectomy was performed due to internal and external bronchial tumor invasion in 29 patients and fissure invasion in 11 patients, and these figures were the opposite of those in the sBL group. In the sBL group, 43 patients were operated due to fissure invasion, whereas the internal and external bronchial tumor invasion was limited to two patients. The bronchial invasion-based indications were significantly higher in the iBL group compared to the fissure invasion-based indications in the sBL group (P < 0.001). Among those who underwent bilobectomy due to bronchial invasion, 21 patients had intermediary bronchus invasion and ten patients had middle lobe bronchus invasion. Among those who underwent bilobectomy due to fissure invasion, 43 patients had minor fissure invasion, and 11 patients had bilobectomy due to tumor invasion in the middle lobe by invading the major fissure.
The mean drainage time was 9.3 ± 7.4 (range: 3-52) days in the overall group. The mean drainage time was 8.3 days in the sBL group compared to 10.3 days in the iBL group, and there was no statistically significant difference between the groups (P = 0.163). When the drainage times were compared considering bilobectomy indication, the mean time was 9.1 days due to bronchial causes, 8.8 days due to fissure-related causes, 15.2 days due to lymph node invasion, and 8.5 days due to vascular causes (P = 0.248).
Forty (43%) patients from the study group developed postoperative complications. The distribution was as follows; prolonged air leak (PAL) in 22 (23.6%) patients, atelectasis requiring bronchoscopy in four (4.3%) patients, cardiac arrhythmia in three (3.2%) patients, bronchopleural fistula in three (3.2%) patients, pneumonia in two (2.1%) patients, empyema in two (2.1%) patients, subcutaneous emphysema in two (2.1%) patients, pulmonary thromboembolism in two (2.1%) patients, and chylothorax in one (1.1%) patient. Re-thoracotomy was performed in four patients, as completion to sleeve resection in two patients, and fistula repair in one patient who developed bronchopleural fistula, and ductus ligation in one patient who developed chylothorax. Two patients died within the first postoperative month (2.1%). Pneumoperitoneum was performed in three of 22 patients who developed PAL. There were no complications in the remaining 53 patients. The bronchus closure was completed using a stapler in two and by suturing in one of three patients who developed bronchopleural fistula, whereas two patients had sBL and the other patient had iBL. Eighteen patients from the sBL group and 22 patients from the iBL group developed complications, and there was a balanced distribution between the groups (P = 0.533). Fourteen patients from the iBL group had PAL compared to eight patients from the sBL group (P = 0.128). When the development of complications was compared considering bilobectomy indication; complications were developed in 16 patients who had bilobectomy due to bronchial causes, 19 patients who were operated due to fissure crossing, three of the patients who were surgically treated due to lymphatic etiology, and two patients who had bilobectomy due to vascular causes, and there was no statistically significant difference between the groups (P = 0.168).
The mean duration of follow-up of the patients was 45.8 months (range: 1-117 months), and 56% of the patients are alive. Intraoperative mortality was not observed in the study group, but two patients died from operation-related complications within the first thirty postoperative days and considered as early mortality. For this reason, the survival calculations were made on 91 patients. As calculated using the Kaplan-Meier survival analysis, the median survival of 91 patients was 40 months and the three-year and five-year survival rates were 64.3% and 59.6%, respectively. When survival was analyzed considering the bilobectomy type, the median survival time was 37 and 41 months for superior and inferior bilobectomy, respectively, and there was no statistically significant difference in between (P = 0.324) (Figure 1). The median survival time was 37, 38, and 49 months for the patients diagnosed with adenocarcinoma, squamous cell carcinoma, and other NSCLC, respectively, and the five-year survival rate was 57.1%, 58.3%, and 75%, respectively (P = 0.687) (Table 2). When the survival of the overall group was analyzed considering lymph node (N) status, the median survival was 49 months in N0, 41 months in N1, and 33 months in N2, and the five-year survival rates were 65.9%, 47.7%, and 45.7%, respectively; there was no statistically significant difference between the groups (P = 0.236).
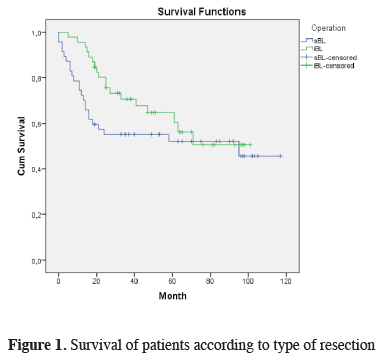 Click Here to Zoom |
Figure 1: Survival of patients according to type of resection |
Table 2: Survival rate of the patients according to resection type, histopathology and n-status
Discussion
Bilobectomy was first performed in 1933, and the limited number of studies conducted afterwards [3,5-6]. Inferior bilobectomy was more frequent in the early studies conducted on this matter, whereas superior bilobectomy has increased in number over time. The rate of inferior bilobectomy was 65% in the series of Keller et al. [4], 86% in the series of Deneuville et al. [6], 84% in the series of Massard et al. [1], 82% in the series of Carbognani et al. [1], and 62% in the series of Kim et al. [7], whereas superior bilobectomy was performed by Icard et al. [2] at a rate of 52% in their series and similarly, at a rate of 52% by Galetta et al. [4] in their series. The numbers of iBL and sBL were close in our series, and sBL was performed in 47 (50.5%) patients and iBL was performed in 46 (49.5%) patients.Among the most important issues that may occur after bilobectomy is the diameter mismatch between lung and pleural cavity, and the pleural and pulmonary complications caused by the remaining lung tissue not being able to fill the thoracic cavity [2,9]. In the literature, the ratio of post-bilobectomy complications varied between 35% and 49% [1,4,6], which is consistent with the ratio (43%) in our series. Several studies have demonstrated that pulmonary complications are more common in the iBL group [1,2,6]. This is attributed to the higher number of lung segments resected in iBL [2]. In the present study, the number of pulmonary complications were higher, but there were no statistical differences between two groups.
One of the most important pulmonary complications that may develop after surgical resection is prolonged air leak in non-small cell lung cancer. The study by Keller et al. stated that the limit for prolonged air leak was 14 days and reported that three patients developed prolonged air leak [5]. Deneuville et al. [6] reported prolonged air leak in ten (6.8%) patients, Kim et al. [8] reported prolonged air leak in eight (9%) patients, Massard et al. [1] reported prolonged air leak in 18 (16%) patients, and Galetta et al. [4] reported prolonged air leak in 22 (15.1%) patients. In the present study, the limit for prolonged air leak was seven days and 22 (23.6%) patients developed PAL. In the study by Massard et al. [1] a higher percentage of prolonged air leak was found in the sBL group, but did not provide any statistical information on this matter. In the present study, prolonged air leak was more common in the iBL group, however there was no statistically significant difference between the groups (P = 0.128).
Bronchopleural fistula is among the major and undesired complications that may develop after pulmonary resections [10]. The rate of incidence for bronchopleural fistula after bilobectomy varied between 0.6% and 8% [4,6]. This rate was 3% in our series. In the study by Vester et al. [11] consisting of a pulmonary resection series of 2000 diseases, nine of the patients who had bilobectomy developed BPF and the bronchial stump was closed by using sutures in all of these patients. In our series, three patients developed BPF and the bronchial stump was closed by using a stapler in two patients and by sutures in one patient. In the study by Deneuville et al. [6], all of 13 patients with BPF were from the inferior bilobectomy group, whereas the superior bilobectomy was performed on two patients and inferior bilobectomy was performed on one of the patients developing BPF in our series.
The mortality rate within the first thirty postoperative days varies from 0.97% to 6.1% in various series [1,2,5,6,8,12]. Icard et al. [2] reported that mortality varied from 3.5% to 6.1% within the first thirty postoperative days in their first series published and highlighted that the mortality within the first thirty postoperative days reduced over time. In the same study, Icard et al. [2] stated that the postoperative mortality of bilobectomies were not statistically different from lobectomies and significantly lower than pneumonectomies, based on his own experience. In our series, two (2.1%) patients died within the first 30 postoperative days due to pulmonary thromboembolism.
The literature review revealed that the five-year survival rate varied between 26% and 58% in patients undergoing bilobectomy for non-small cell lung cancer [1,2,4,6,8]. In our series, the median survival of the overall group was 40 months, and the five-year survival was 59.6%. The study by Galetta et al. [4] found that the five-year survival rate was 43% and 76.2% in patients who had superior and inferior bilobectomy, respectively, and reported a significant difference between the groups. In the present study, the five-year survival rate was 60.4% in patients from the superior bilobectomy group and 54.4% in the patients from the inferior bilobectomy group. There is no significant difference between these two groups.
The bilobectomy series in the literature revealed that, the five-year survival rate varied from 34% to 74.5% in N0 disease and this rate varied between 8% and 40% in N2 disease [2,4,8]. The study by Kim et al. [8] showed that the five-year survival rates were 62%, 35%, and 8% in N0, N1, and N2 disease, respectively, which was significant. In the present study, the median survival and five-year survival rates were 49 months and 65.9% in N0 disease, 41 months and 47.7% in N1 disease, and 33 months and 45.7% in N2 disease, respectively, and there was no statistically significant difference between the groups despite the reduced values.
As a conclusion bilobectomy leads to a substantial amount of parenchymal loss in the right lung, but it is a procedure that should be performed under the necessary conditions. The present study demonstrated that both inferior and superior bilobectomy can be performed at acceptable rates. It is obvious that performing bilobectomy under proper indications would result in good outcomes for lung cancer patients.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Reference
1) Massard G, Dabbagh A, Dumont P, Kessler R, Roeslin N, Wihlm JM, et al. Are bilobectomies acceptable procedures? Ann Thorac Surg 1995; 60: 640-5.
2) Icard P, Heyndrickx M, Galateau-Salle F, Rosat P, Lerochais JP, Gervais R, et al. Does bilobectomy offer satisfactory long-term survival outcome for non-small cell lung cancer? Ann Thorac Surg 2013; 9: 1726-33.
3) Churchill ED. The surgical treatment of carcinoma of the lung. J Thorac Surg 1933; 2: 254-66.
4) Galetta D, Solli P, Borri A, Petrella F, Gasparri R, Brambilla D, et al. Bilobectomy for lung cancer: analysis of indications, postoperative results, and long-term outcomes. Ann Thorac Surg 2012; 93: 251-7.
5) Keller SM, Kaiser LR, Martini N. Bilobectomy for bronchogenic carcinoma. Ann Thorac Surg 1988; 45: 62-5.
6) Deneuville M, Regnard JF, Coggia M, Rojas-Miranda A, Dartevelle P, Levasseur P. The place for bilobectomy in bronchogenic carcinoma. Eur J Cardiothorac Surg 1992; 6: 446-51.
7) Carbognani P, Tincani G, Solli P, Galimberti A, Cattelani L, Bobbio A, et al. The bilobectomies for lung cancer. J Cardiovasc Surg 2001; 42: 421-4.
8) Kim AW, Faber LP, Warren WH, Shah ND, Basu S, Liptay MJ. Bilobectomy for non-small cell lung cancer: a search for clinical factors that may affect perioperative morbidity and long-term survival. J Thorac Cardiovasc Surg 2010; 139: 606-11.
9) Gomez MT, Jimenez MF, Aranda JL, Rodriguez M, Novoa NM, Varela G. The risk of bilobectomy compared with lobectomy: a retrospective analysis of a series of matched cases and controls. Eur J Cardiothorac Surg 2014; 16: 72-75.
10) Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Semin Thorac Cardiovasc Surg 2001; 13: 3-7.






