2Deparment of Radiology, Bakırköy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey
3Department of Chest Diseases, Yedikule Chest Diseases and Thoracic Surgery Training and Research Hospital, Istanbul, Turkey DOI : 10.26663/cts.2024.024
Summary
Background: The aim of this study is two-fold; first is to investigate the determinants of malignancy from the clinical and radiological results of patients operated on solitary pulmonary nodules (SPN) and second is to determine the SUVmax threshold value for predicting malignancy based on nodule size. While the literature tends to focus on many variables as a group for understanding the SPNmalignancy link, this study asks whether we could weigh size more compared to other factors to decide on malignancy potential. The fact that the incidental finding of SPNs is on rise means timely prediction and cure are within our reach.Materials and Methods: Patients operated in our clinic due to SPN between January 2018 and December 2022 were categorized into two groups according to their histopathologic diagnoses as malignant and benign. The clinical and radiological characteristics of the two groups were then analysed.
Results: Among the 522 patients included in the study, 385 (74%) were male and the mean age was 61 ± 10.5 years. The high SUVmax value of the nodule and the presence of lobulation and spiculation were found to be determinant factors for malignancy (p<0.05, for all). In addition, SUVmax values of 1.75 for nodules <1 cm in diameter, 2.24 for nodules between 1-2 cm, and 2.55 for nodules >2 cm were determined as malignancy predictors.
Conclusions: High SUVmax and the presence of lobulation and spiculation are positive predictive factors for malignancy in SPNs. We believe that the SUVmax value is discretely (by itself) critical in predicting malignancy according to the diameter of the nodule. As size is a relatively straightforward variable to measure and SPN is largely curable with timely intervention, the capacity of size to be a predictor would be highly convenient. In this respect, to better exploit the SUVmax indicator in practice during prognosis, more sensitive cut-off values in an ascending/descending order of the module diameter could be defined.
Introduction
A solitary pulmonary nodule (SPN) is a radiologic manifestation defined as a round or oval lesion with a diameter of ≤3 cm, unaccompanied by atelectasis, pneumonia, or lymphadenopathy, and fully separated from the surrounding intact lung tissue [1,2]. SPNs are classified as either solid or subsolid nodules while subsolid nodules are further subdivided into part-solid or ground glass nodules (GGN) [2]. With the increased use of high-resolution computed tomography, our capability to observe solitary pulmonary nodules (SPNs) increased along with the frequency of actual findings of SPNs. [3]. According to low-dose computed tomography (CT) screening tests, the incidence of nodular lesions is 19.5%, and most of these nodules are benign. A malignancy rate of 3% is observed in nodules detected during screening, while malignancy is observed at rates ranging up to 60% in nodules detected following clinical symptoms [4].Functional imaging techniques such as Positron Emission Tomography-Computed Tomography (PET-CT) are essential due to the well-known, widespread complications of radiologic imaging techniques encountered in distinguishing the malignant from the benign [5]. PETCT containing 18F-fluorodeoxyglucose (FDG) is particularly effective in differentiating between benign and malignant SPNs [6]. Chest radiography, CT, and PET-CT have been proven to complement each other in diagnosing lung cancer [7]. The accurate classification of SPNs can be achieved by considering patient risk factors and radiologic features together in a holistic manner. Identifying SPN enables early diagnosis and treatment in patients with malignant tumors and thus prevents unnecessary, undesirable intervention in benign cases.
In the present study, predictors of malignancy in SPNs were investigated in a large study cohort.
Methods
The present study is a retrospective, single-center, observational study designed in a teaching and research hospital, which is a reference center for chest diseases and thoracic surgery. The patients who were operated on SPNs were reviewed.
Organization of the thoracic surgery clinic and operations
In our clinic, operation decisions and treatment management policies are, in their entirety, under the responsibility
of surgery councils. Thoracic surgeons and
pulmonologists are the main members of these councils.
Other regular participants include specialists from
oncology, radiology, radiation oncology, nuclear medicine,
and pathology.
Study design
The study included 522 patients who underwent surgery
due to SPN between January 2018 and December 2022.
Patients with a history of malignancy within the last 5
years, multiple nodules, and histopathologic diagnosis
of metastasis were excluded from the study.
Demographic characteristics of patients, size, and location of nodules, PET-CT FDG uptake, type of surgical resection, and final histopathologic diagnosis were recorded. Density was calculated according to the Hounsfield Unit (HU) scale.
Radiological images of all patients were evaluated by an inter-branch joint council of three chest surgeons, one pulmonologist, and one radiologist. Solid component, lobulation, spiculation, air bronchogram, pleural indentation, calcification, and cavitation conditions were recorded.
Patients were categorized into two groups according to their histopathologic diagnoses, namely, malignant and benign. Clinical and radiologic results of all patients were compared between the two groups. This study was approved by the Sureyyapasa Chest Diseases and Thoracic Surgery Training and Research Hospital Ethics Committee. (03.03.2023, No: 116.2017.R-269).
Statistical Analysis
All data are expressed as mean ± standard deviation.
A Chi-square and Student’s t-test were used to evaluate
the data obtained from inter-group comparison. The
diagnostic values of the statistically significant parameters
were evaluated in accordance with the sensitivity,
specificity, positive predictive value (PPV) and negative
predictive value (NPV), and from the cut-off values
obtained from receiver operating characteristic (ROC)
curves. All statistical analysis was carried out using statistical
software package system (SPSS for Windows,
version 16.0; SPSS Inc., Chicago, IL, USA). A p value
of < 0.05 was considered significant.
Results
Among the 522 patients included in the study, 385 (74%) were male and the mean age was 61 ± 10.5 years. The most common location of SPN was the upper lobe of the right lung (34%). The mean nodule size was 1.858 ± 0.6 cm (0.5-3.0), and the mean SUVmax value was 5.4 ± 4.18 (NA-19) (Table 1). Among the patients, 360 (69 %) underwent video-assisted thoracoscopic surgery and 162 (31%) underwent thoracotomy. The pathology result was reported as 326 (62.5%) malignant and 196 (37.5%) benign. The most common malignant histopathological diagnosis was adenocarcinoma (210, 64%) while the most common benign diagnosis was chronic inflammation and fibrosis.The joint council retrospectively analyzed PET-CT images of the patients, and the mean nodule density was calculated as -14.9 ± 115 (NA-19). The number of solid nodules was 343 (65%). Lobulation was observed in 164 (31%), spiculation in 153 (29%), and pleural indentation in 222 (42%) (Table 1).
Table 1. Clinical and radiological features of 522 patients.
According to the comparison between malignant and benign patient groups, the mean age was significantly higher in the malignant patient group (63.8 ± 8.5 p = 0.0001). There were 257 (78%) male patients in the malignant patient group (p = 0.001). While the side location of the nodule was similar between the two groups (p = 0.855), upper lobe location was significantly more prevalent in the malignant group (p = 0.013). The mean nodule size was statistically higher in the malignant group (<0.0001), and the size >2 cm was significant for malignancy (<0.0001). The mean SUVmax was higher in the malignant group with statistical significance (6.81 ± 4.24, 3.18 ± 2.91, p < 0.0001). The density values of the nodule were similar between the two groups (p = 0.514) (Table 1).
The incidence of malignancy was higher in solid lesions compared to subsolid lesions and GGOs (p = 0.023). Lobulation and spiculation were statistically more common in the malignant patient group (p = 0.032, p < 0.001) while air bronchogram, pleural indentation, calcification, and cavitation findings were similar in both groups (p > 0.05) (Table 1).
When the malignancy-wise significant parameters were evaluated by multivariate analysis, the high SUVmax value of solitary pulmonary nodule and the presence of lobulation and spiculation were found to be positive predictive factors for malignancy (p < 0.05) (Table 2). Among these, SUVmax could further be distinguished with more robust predictive prowess for two reasons. First, because it is measurable and hence practical to earmark malignancy. Second, rather than being an either/or (either present or absent) factor such as lobulation, SUVmax is sensitive to the patient’s overall conditions, implying that its size along with other factors promises an accurate diagnosis for the individual patient.
Table 2. Multivariate analysis of potential factors for predicting malignancy.
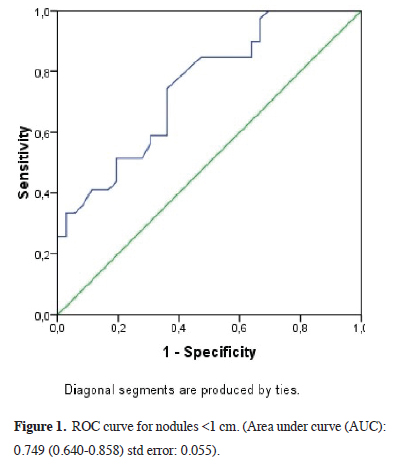
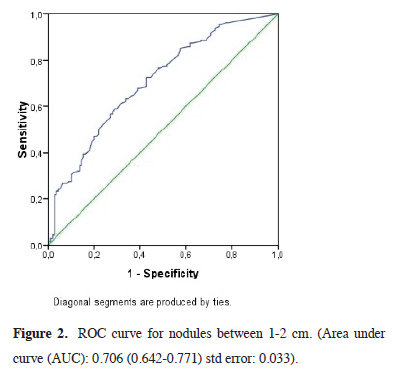
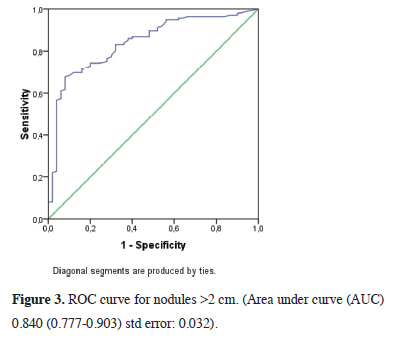
In comparing the mean SUVmax values according to nodule size in malignant and benign patients, we observed that the SUVmax value was markedly higher in the malignant group with a statistical significance (p < 0.0001) (Table 3). The SUVmax value of 1.75 (AUC: 0.749 (0.640- 0.858) std error: 0.055) was determined as the malignancy marker for nodules smaller than 1 cm with 85% sensitivity and 42% specificity (Figure 1) and the SUVmax value of 2.24 (AUC: 0.706 (0.642-0.771) std error: 0.033) was determined as the malignancy marker for nodules between 1 and 2 cm with 85% sensitivity and 43% specificity (Figure 2). In addition, the SUVmax value of 2.55 (AUC: 0.840 (0.777-0.903) std error: 0.032) was determined as the malignancy marker for nodules >2 cm with 91% sensitivity and 48% specificity (Figure 3).
Table 3. The average PET-SUVmax values to predict malignancy according to nodule diameter.
 Click Here to Zoom |
Figure 1: ROC curve for nodules <1 cm. (Area under curve (AUC): 0.749 (0.640-0.858) std error: 0.055). |
 Click Here to Zoom |
Figure 2: ROC curve for nodules between 1-2 cm. (Area under curve (AUC): 0.706 (0.642-0.771) std error: 0.033). |
 Click Here to Zoom |
Figure 3: ROC curve for nodules >2 cm. (Area under curve (AUC) 0.840 (0.777-0.903) std error: 0.032). |
Discussion
The present study analyzes malignancy predictors of SPNs in depth in a large study group. While the study yielded lobulation and spiculation as independent risk factors, the more remarkable result of the study was establishing the statistical overlaps/similarities among air bronchogram, pleural indentation, calcification, and cavitation rates. These overlaps led us to search for a more accurate determinant for malignancy. It is widely acknowledged that malignancy risk is not easily tested and resolved; nodule-specific as well as patient-specific factors complicate the prognosis process, especially the decision about patient surveillance. In this respect, our finding of PET-BT cut-off based on nodule size could also imply that both patient-specific factors and nodulerelated elements are inherent SUVmax. This, in turn, could facilitate decision making.A comprehensive clinical history is essential in the evaluation of an SPN. A successful classification of the patients presenting with SPN can be achieved by considering patient risk factors together with radiologic features. Such evaluation secures timely diagnosis and thus successful treatment in patients with malignant tumors while minimizing unnecessary intervention in otherwise benign cases [8].
Several clinical studies have reported that age, history of smoking, and cancer background are associated with a high risk of malignancy in patients with SPN [9,10]. Erdogdu et al found that age above 61 was an independent risk factor for malignancy [8]. In our study, the mean age was significantly higher in malignant patients in one-way analyses; however, it was similar between the two groups in multi-way analyses. It could thus be proposed that the predictive prowess of age may be ambiguous.
On computed tomography (CT) scans, the main features defining the nodule are: size, shape, density, presence of calcification, and growth rate [3,11]. Nodular lesions with pleural recession, lobule contours, and spicular extensions are more likely to be malignant. Several studies have reported spicular extensions as markers of malignancy in nodules [8,12]. While spicular extension was also observed as a marker of malignancy in our study, the presence of lobulation was also determined as a marker. Ambrossini et al. reported that aHU index of ≥15 was a predictive factor for malignancy [13]. In our study, density values were found to be similar between the two groups. Calcified nodules are generally benign [14]. Yet, up to 6-10% of primary lung cancers exhibit calcification on CT [15]. Benign nodules, especially hamartomas, exhibit diffuse calcification patterns in addition to popcornlike pathognomonic calcification. Punctate and eccentric calcification patterns are also screened in malignant nodules [15]. Cavitation occurs in both benign and malignant conditions [16]. While air bronchograms are frequently encountered in infectious conditions, they are especially present/common in malignant nodules in SPN [17]. Similar rates of calcification, cavitation, and air bronchograms were observed in both groups in our results. Again, the similarity in both groups alarmed us that we needed a safer predictive factor, delving deeper into the different values for the two groups to distinguish a factor that could have a causal relationship to malignancy.
The location of SPNs may also help determine the risk of malignancy. Some studies have reported that the upper lobe is associated with an increased risk of malignancy and that particularly right upper lobe commands the highest malignancy rate with 45% of all malignant nodules [18]. On the other hand, another group of studies have concluded that location may not constitute a significant malignancy marker [8,12]. In our study, in line with the latter group of studies, although upper lobe location was significantly more prevalent in the malignant group, it was not considered a malignancy marker in multidimensional analyses.
While some studies have reported a positive correlation between the diameter of the nodule and malignancy, others have not observed such a phenomenon [19,20]. According to the studies of the Fleischner Society, approximately 80% of lesions larger than 20 mm were identified as malignant, while 1% of lesions 2-5 mm in size were identified so [21]. A further research demonstrated that the probability of malignancy ranged from 6% in nodules with a size of 5-10 mm to 64% in nodules larger than 20 mm [2]. The volume doubling time (VDT), the time it takes for the nodule to double in volume or increase in diameter by 26%, can also serve as a measure for assessing the growth of the SPN and predicting benignity/ malignancy [22]. In this sense, numerous studies have reported a mean duration of VDT of 139 days for lung malignancy [23]. In our study, the mean nodule size was statistically greater in the malignant group, and a size of >2 cm was significant for malignancy.
With the popularity and prevalence of screening examinations in recent years, several models have been developed to help determine the risk of malignancy. In this sense, the Mayo model reported six independent risk factors: age, smoking history, history of extrapulmonary tumor, maximum diameter, nodule location, and spiculation [13]. The Veterans Affairs (VA) model, on the other side, reported age, smoking history, smoking cessation time, and nodule diameter as independent risk factors for malignancy [14]. According to the Peking University People's Hospital (PKUPH) model, the independent risk factors constituted age, nodule diameter, family history of cancer, and spiculation [12]. These models are effective for decision-making in the case of suspected malignancy, yet their results may produce rather approximate and vague pictures. As more clarity is a must for individual evaluations and decisions on the mode of patient surveillance, we asked whether a concrete factor could be separated.
Functional imaging capabilities such as PET-CT are necessary due to the complications of radiological imaging techniques in differentiating between malignant and benign cases [5]. The FDG PET-CT method provides morphological and metabolic information about the lesions, enabling semi-quantitative evaluation by calculating the maximum standardized uptake value (maxSTD) besides visual evaluation [24]. Considering the threshold value for maxSTD as 2.5, the sensitivity and specificity of FDG PET-CT examination in detecting malignant lesions were determined as 95.1% and 50.0%, respectively [25]. The FDG PET-CT has a high level of accuracy for diagnosing malignancy in nodules, and a recent meta-analysis reported a sensitivity and specificity of 89% and 70%, respectively [26]. In parallel, in our case, the mean SUVmax was found higher in the malignant group with a statistical significance.
PET-CT plays an essential role in characterizing the nature of pulmonary nodules, yet its predictive power is reduced in nodules smaller than 1 cm [27]. The sensitivity of PET-CT was reported as 69% for nodules with a diameter of 5-10 mm and 95% for nodules larger than 10 mm [28]. In active granulomatous infections, most commonly tuberculosis, high FDG values are known to produce false positive results in the differential diagnosis of malignancy [29]. In addition, it may produce false negative results for carcinoid tumors, adenocarcinoma subtypes, and lung tumors smaller than 1 cm [6]. Amidst these errors, some modifications have been made to increase the sensitivity and specificity of FDG PET-CT in evaluating SPNs. Matthies et al reported a 20% increase in sensitivity in detecting malignant lesions using the dual-phase method [30]. On the other hand, Fletcher et al concluded in a meta-analysis that the dual-phase method had zero contribution to the differential diagnosis of malignant lesions [31]. Another type of modification was performed in clinical trials using a ranking scale comparing the SUVmax cut-off value to the mediastinal blood pool. It was reported that normalization of SUVmax to blood pool or liver uptake had high diagnostic accuracy for detecting malignancy in SPNs, with AUCs of 0.90 for both liver and blood uptake rates [32].
Several clinical trials have identified malignancy markers of SPNs by evaluating CT features, patient risk factors, and F-18 FDG PET-CT results. In a study including 223 patients with a radiologic diagnosis of the solitary pulmonary nodule, Erdogdu et al demonstrated that an SUVmax value greater than 2.5, spicular extension, and age above 61 years were independent markers of malignancy [8]. Lopez et al. also revealed that SUVmax value and age were independent variables in predicting malignancy [33]. In a recent multi-center study on 355 patients, Weir-McCall et al reported that SUVmax was the most accurate technique in diagnosing solitary lung nodules; however, they also showed that the diagnostic thresholds should be modified according to nodule size. They determined the optimal thresholds for SUVmax according to lesion size and set them as 1.75 for lesions <12 mm, 2.55 for lesions ranging between 12 and 16 mm, and 3.6 for lesions >16 mm [34]. In our study, we propose the following nodule size-SUVmax pairs: the SUVmax value of 1.75 in nodules smaller than 1 cm; 2.24 in nodules between 1 and 2 cm; 2.55 in nodules greater than 2 cm. These proposed thresholds, rather easy to assess, would provide a timely evaluation and save various treatment costs by excluding benign cases. Avoiding the needless intervention in the patients’ daily lives would also be a plus.
Our study reports the following important limitations: being retrospective and being single-centered. On the other hand, our study has been conducted on a large group in a specialist pulmonary diseases center where our patients are fully examined and where all procedures are followed in detail, from detection to the probable operation. Therefore, the patient population here is a special focus group. Another strength is the rich size of the population along with the range of patient profiles, rendering our findings robust.
In conclusion, the presence of radiological spiculation and lobulation along with high PET-CT SUVmax values strongly signal malignancy, making these variables critical factors for deciding on the follow-up or treatment of SPNs. The increased technological capacity of successful detection of millimetric lesions with the growing use of high-resolution computed tomography could be harnessed for better test/decision criteria. In this respect, the standard SUVmax threshold value, in its extant form, may be insufficient for diagnostic prediction in these lesions and prone to spurious results. The SUVmax diagnostic threshold value should be updated to be set in a step-wise manner according to nodule sizes. The inclusion of such a sensitive predictive element in evaluations may lead to better decisions early on.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect
to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research
and/or authorship of this article.
Ethics approval
This study was approved by the Sureyyapasa Chest Diseases
and Thoracic Surgery Training and Research Hospital
Ethics Committee. (03.03.2023, No: 116.2017.R-269).
Authors' contributions
MA,AU,FTA; conception and design, VB,EEK,SB;
administrative support, AU,EY,MA; provision of study
materials or patients, MA,AU,ŞB,SB; collection and
assembly of data, FTA,MA,ŞB,SE; data analysis and
interpretation, MA,AU; manuscript writing. All authors
have read and approved the final manuscript.
Reference
1) Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. American
College of Chest Physicians. The solitary pulmonary nodule.
Chest 2003; 123: 89-96.
2) Larici AR, Farchione A, Franchi P, Ciliberto M, Cicchetti G,
Calandriello L et al. Lung nodules: size still matters. Eur Respir
Rev 2017; 26: 1-16.
3) Brandman S, Ko JP. Pulmonary nodule detection, characterization,
and management with multidetector computed tomography.
J Thorac Imaging 2011; 26: 90-105.
4) Silva M, Pastorino U, Sverzellati N. Lung cancer screening
with low-dose CT in Europe: strength and weakness of diverse
independent screening trials. Clin Radiol 2017; 72: 389-400.
5) Yilmaz F, Tastekin G. Sensitivity of 18F-FDG PET in evaluation
of solitary pulmonary nodules. Int J Clin Exp Med 2015; 8: 45–51.
6) Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Uno
K. Visual and semiquantitative analyses for F-18 fluorodeoxyglucose
PET scanning in pulmonary nodules 1 cm to 3 cm in
size. Ann Thorac Surg 2005; 79: 984-8.
7) Bombardieri E, Buscombe J, Lucignani G, Schober O. Advances
in Nuclear Oncology Diagnosis and Therapy. Lung Cancers
2007; 5: 62-80.
8) Erdogdu E, Ozkan B, Duman S, Agkoc M, Erturk S.M, Kara M
et al. Predictors of Malignancy in Patients with Solitary Pulmonary
Nodules Undergoing Pulmonary Resection. Clin. Respir J
2022; 16: 361-8.
9) Schultz EM, Sanders GD, Trotter PR, Patz Jr EF, Silvestri GA,
Owens DK et al. Validation of two models to estimate the probability
of malignancy in patients with solitary pulmonary nodules.
Thorax 2008; 63: 335-41.
10) Mery CM, Pappas AN, Bueno R, Mentzer SJ, Lukanich JM,
Sugerbaker DJ et al. Relationship between a history of antecedent
cancer and the probability of malignancy for a solitary
pulmonary nodule. Chest 2004; 125: 2175-81.
11) Mikita K, Saito H, Sakuma Y, Kondo T, Honda T, Murakami
S et al. Growth rate of lung cancer recognized as small solid
nodule on initial CT findings. Eur J Radiol 2012; 81: 548-53.
12) Xiao F, Liu D, Guo Y, Shi B, Song Z, Tian Y et al. Novel and convenient
method to evaluate the character of solitary pulmonary
nodule-comparison of three mathematical prediction models and
further stratification of risk factors. PLoS ONE 2013; 8: e78271.
13) Swensen SJ, Viggiano RW, Midthun DE, Müller NL, Sherrick
A,Yamashita K et al. Lung nodule enhancement at CT: multicenter
study. Radiology 2000; 214: 73-80.
14) Li Y, Wang J. A mathematical model for predicting malignancy
of solitary pulmonary nodules. World J Surg 2012; 36: 830-5.
15) Khan AN, Al-Jahdali HH, Allen CM, Irion KL, Al Ghanem S,
Koteyar SS. The calcified lung nodule: what does it mean? Ann
Thorac Med 2010; 5: 67-79.
16) Truong MT, Ko JP, Rossi SE, Rossi I, Viswanathan C, Bruzzi
JF et al. Update in the evaluation of the solitary pulmonary
nodule. Radiographics 2014; 34: 1658-79.
17) Algin O, Gokalp G, Topal U. Signs in chest imaging. Diagn
Interv Radiol 2011; 17: 18-29.
18) Horeweg N, van Rosmalen J, Heuvelmans MA, van der Aalst
CM, Vliegenthart R, Scholten ET et al. Lung cancer probability
in patients with CT-detected pulmonary nodules: a prespecified
analysis of data from the NELSON trial of low-dose CT screening.
Lancet Oncol 2014; 15: 1332-41.
19) Yang D, Li Y, Liu J, Jiang G, Li J, Zhao H et al. Study on
solitary pulmonary nodules: correlation between diameter and
clinical manifestation and pathological features. Zhongguo Fei
Ai Za Zhi 2010; 13: 607-11.
20) Nguyen NC, Kaushik A, Wolverson MK, Osman MM. Is there
a common SUV threshold in oncological FDG PET/CT, at least
for some common indications? A retrospective study. Acta Oncologica
2011; 50: 670-77.
21) Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller
NL, Remy J et al. Fleischner Society: glossary of terms for
thoracic imaging. Radiology 2008; 246: 697-722.
22) Bailly P, Bouzerar R, Shields T, Meyer ME, Daouk J. Benefits
of respiratory-gated 18 F-FDG PET acquisition in lung disease.
Nucl Med Commun 2018; 39: 44-50.
23) Walter JE, Heuvelmans MA, de Jong PA, Vliegenthart R, van
Ooijen PMA, Peters RB et al. Occurrence and lung cancer
probability of new solid nodules at incidence screening with
low-dose CT: analysis of data from the randomised, controlled
NELSON trial. Lancet Oncol 2016; 17: 907-16.
24) Czernin J, Allen-Auerbach M, Nathanson D, Herrmann K.
PET/CT in Oncology: Current Status and Perspectives. Curr
Radiol Rep 2013; 1: 177-90.
25) Hou S, Lin X, Wang S, Shen Y, Meng Z, Jia Q et al. Combination
of positron emission tomography/computed tomography
and chest thin-layer high resolution computed tomography for
evaluation of pulmonary nodules: Correlation with imaging
features, maximum standardized uptake value, and pathology.
Medicine (Baltimore) 2018; 97: e11640.
26) Li Z-Z, Huang Y-L, Song H-J, Wang Y-J, Huang Y. The value
of 18 F-FDG-PET/CT in the diagnosis of solitary pulmonary
nodules: a meta-analysis. Med (Baltimore) 2018; 97: e0130.
27) Ambrosini V, Nicolini S, Caroli P, Nanni C, Massaro A, Marzola
MC et al. PET/CT imaging in different types of lung cancer:
an overview. Eur J Radiol 2012; 81: 988-1001.
28) Bakheet SM, Saleem M, Powe J. F-18 FDG chest uptake in lung
inflammation and infection. Clin Nucl Med 2000; 25: 273-8.
29) Li Y, Su M, Li F, Kuang A, Tian R. The value of 18F-FDG-PET/
CT in the differential diagnosis of solitary pulmonary nodules
in areas with a high incidence of tuberculosis. Ann Nucl Med
2011; 25: 804-11.
30) Matthies A, Hickeson M, Cuchiara A, Alavi A. Dual time point
18F FDG PET for the evaluation of pulmonary nodules. J Nucl
Med 2002; 43: 871 5.
31) Fletcher JW, Kymes SM, Gould M, Alazraki N, Coleman RE,
Lowe VJ et al. A comparison of the diagnostic accuracy of
18FFDG PET and CT in the characterization of solitary pulmonary
nodules. J Nucl Med. 2008; 49: 179-85.
32) Evangelista L, Cuocolo A, Pace L, Mansi L, Del Vecchio S,
Miletto P et al. Performance of FDG-PET/CT in solitary pulmonary
nodule based on pre-test likelihood of malignancy: results
from the ITALIAN retrospective multicenter trial. Eur J
Nucl Med Mol Imaging 2018; 45: 1898-907.






