2Department of Thoracic Surgery, Faculty of Medicine, Dokuz Eylül University, İzmir, Turkey
3Department of Cardiovascular Surgery, Faculty of Medicine, Dokuz Eylül University, İzmir, Turkey DOI : 10.26663/cts.2025.001
Summary
Background: In non-small cell lung cancer (NSCLC), pulmonary artery, pulmonary vein, pericardium, or atrium involvement is not considered as an inoperability criterion, although it negatively affects the disease prognosis. In these patients accepted as local disease, intrapericardial pneumonectomy can be performed to leave a safe surgical margin. In this study, patients diagnosed with NSCLC who underwent intrapericardial pneumonectomy were subjected to clinical evaluation.Materials and Methods: We retrospectively evaluated NSCLC patients who were diagnosed as T3- T4 and N0-N1 with preoperative invasive staging, who responded to neoadjuvant treatment for T4N2 disease, or who were considered persistent N2 and underwent intrapericardial pneumonectomy for intrapericardial pulmonary artery (PA) and pulmonary vein (PV) invasion.
Results: Thirteen patients underwent intrapericardial left pneumonectomy and 7 patients underwent intrapericardial right pneumonectomy. The median overall survival (OS) value of the patients included in the study was found to be 22.3 months, 95% CI (Confidence Interval) (5.6-39.0). The 1-month postoperative survival rate was 90%, the 6-month survival rate was 88%, the 1-year survival rate was 84%, and the 3-year survival rate was 35%. The assessment of the 5-year survival rate was conducted among 13 cases who successfully completed the 5-year follow-up period, revealing a median OS of 35,0 months and a 5-year survival rate of 23%. Patients who did not receive pre-op neoadjuvant therapy, patients who underwent right pneumonectomy and patients with squamous histopathology had a lower median OS and higher HRs for mortality.
Conclusions: In NSCLC patients who underwent intrapericardial pneumonectomy, right pneumonectomy, lymph node involvement, lymphovascular invasion, and lack of neoadjuvant treatment were evaluated as poor prognostic factors.
Introduction
Lung cancer is the primary cause of cancer-related deaths, 80% of which are non-small cell lung cancers (NSCLC). Treatment includes surgery, chemotherapy, radiotherapy, immunotherapy options, and a combination of these options. The most effective treatment option is surgery, and only 15-25% of NSCLC cases are operable at the time of diagnosis. The basis of surgical treatment is anatomical resection and lymph node dissection. 30% of the cases are seen as locally advanced disease at the time of diagnosis [1-3]. However, in patients with locally advanced disease, invasion of the pericardium, intrapericardial vascular structures and even atrium is not accepted as an inoperability-irresectability criterion, and systemic treatment without surgery is preferred by some centers. Due to the necessity of complete resection, intrapericardial pneumonectomy can be performed in these cases [4]. Intrapericardial involvement of pulmonary arteries and veins in thorax CT images is an indication for elective surgery. Conditions requiring intrapericardial pneumonectomy are; cases where the pulmonary artery and vein are involved intrapericardially, cases where pericardial involvement is present, after radiotherapy applications to central tumors, hilar lymph nodes are involved to a degree that does not allow dissection, adhesions that prevent dissection due to previous infections or surgeries.The aim of this study is to clinically evaluate the surgical results in our series of 20 cases diagnosed with NSCLC and undergoing en-bloc resection. In addition, it is to show that cardiovascular surgical techniques are necessary in thoracic surgery and that resection can be easily performed with the help of these techniques.
Methods
In our study which was conducted in our clinic between 2016 and 2020, patients who were accepted as T3-T4 and N0-N1and operable according to the International Association Study of Lung Cancer (IASLC) 2017 classification with no mediastinal lymph node involvement and distant organ metastasis, or T4N2 patients that received a response to neoadjuvant treatment and were considered persistent N2, or patients who had intrapericardial pulmonary artery (PA), pulmonary vein (PV) invasion and underwent intrapericardial pneumonectomy due to the diagnosis of NSCLC were included. Main exclusion criteria are the presence of N2 disease in oncological principles, small cell carcinoma, and distant organ metastasis. The endpoint of the study is the absence of any other cardiovascular and intrathoracic T4 involvement except for the PA. All cases were operated on by the same thoracic surgeon and cardiovascular surgeon. Ethics committee approval was obtained from Dokuz Eylül University Non-invasive Research Ethics Committee with protocol number 2022-CREC-22-41.All cases were evaluated preoperatively for cardiac and pulmonary reserve by physical examination, routine blood tests, chest radiography, fiber optic bronchoscopy (FOB), and pulmonary function test (PFT). Thoracic computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) were used in clinical staging in cases with suspected diaphragmatic and atrium invasion. Patients with forced expiratory volume in 1 second (FEV1) value over 2 liters were found suitable for the operation. Patients with an FEV1 value of less than 2 liters (80%) were re-evaluated with carbon monoxide diffusion capacity measurement (DLCO), ventilation perfusion scintigraphy, and cardiopulmonary exercise tests. Endobronchial ultrasonography (EBUS) or mediastinoscopy and lymph node sampling were performed to exclude metastasis in cases with mediastinal lymph nodes with a short axis longer than 1 cm in the thorax CT performed for histopathological staging and/or mediastinal lymph nodes with suspicious FDG uptake on PET/CT.
Thoracotomy was performed in the 5th intercostal space in all cases under general anesthesia, with double lumen endobronchial intubation in the lateral position after appropriate sterilization measures. The lung was pushed posteriorly, and the pericardium was suspended with the help of a clamp, and the pericardium was opened by preserving the phrenic nerve at the level of the pulmonary vein. The pericardial incision was advanced to the pulmonary artery until the safe surgical margin was reached, and the pulmonary artery, superior and inferior pulmonary vein, and, when necessary, and lung anatomical resection and detailed mediastinal lymph node dissection were performed. In cases of uncertainty regarding surgical margins, an intraoperative frozen section was performed. In all cases, the adjacent viable tissues were sutured along the stapler line with 4-0 polyproplene sutures to protect the bronchial stump. Furthermore, to mitigate the risk of potential cardiac herniation, closure of the pericardial defect was accomplished utilizing polyproplene mesh.
Statistical Analysis
Statistical analyzes were performed using SPSS 19.0 software
(SPSS Inc., Chicago, IL, USA). Continuous variables
are expressed as mean ± SD, categorical variables
are expressed as n (%). Kaplan-Meier method and log
rank test were used for survival analyses. COX regression
analysis was used to determine risk factors for mortality.
The results are presented with 95% CI and the statistical
significance level was determined as p < 0.05 in all tests.
Results
A total of 20 cases, 15 males and 5 females, were included in the study. The average age of the patients was determined as 59.1 ± 10.4 years (44-79, median 57.5 years) and the average tumor diameter was 50.3 ± 28.2 mm (12.0-140.0), median 47.5 mm). The histopathological diagnosis was detected as squamous cell carcinoma (60%) in 12 of the patients, adenocarcinoma in 5 (25%), large cell neuroendocrine carcinoma in 2 (10%), and adenoid cystic carcinoma (5%) in 1 of the patients (Table 1).Table 1: Demographic data and clinical characteristics of the patients.
Intrapericardial left pneumonectomy was performed in 13 patients, and intrapericardial right pneumonectomy was performed in 7 patients. Surgical margin positivity was observed in 20% of the patients (Table 1). Eleven of the patients with preoperative stages IIIA and IIIB received neoadjuvant CT, RT, or concurrent CRT. While 33% of patients who did not receive neoadjuvant treatment were still alive, 66% died. After pneumonectomy, 10 patients were evaluated as stage IIIA, 5 patients as stage IIIB, 2 patients as stage IIB, 2 patients as stage IB and 1 patient as stage IA. When evaluated according to postoperative lymph node involvement, 7 patients were N2, 5 patients were N1, and 8 patients were N0 (Table 1). Four of the patients evaluated as Stage IIB, IA, IB received neoadjuvant CT+RT or simultaneous CRT. Among these patients, two patients with stage IIIB at the time of diagnosis were evaluated as having regressed to IA and IB after treatment, and two patients with IIIA had regressed to IIB and IB.
As to pathological stage, 40% of the patients had pathological stage of N0, 25% had N1, and 35% had N2 pathological stage. When the surviving patients were evaluated according to lymph node, 20% were N0 and 80% were N1 patients. Again, according to the postoperative lymph node involvement of the patients with left pneumonectomy, 4 were evaluated as N2, 4 as N1, 5 as N0, while in right pneumonectomy, 3 were evaluated as N2, 1 as N1, 3 as N0. Demographic and clinical characteristics of the patients are presented in Table 1.
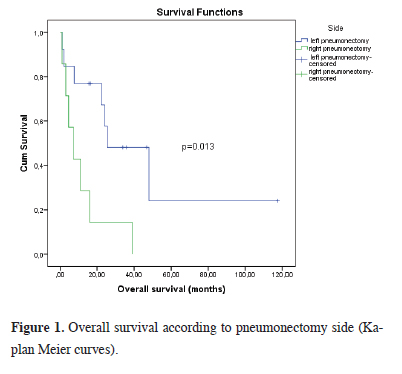
During the retrospective evaluation, 6 (30%) of the patients were alive, while 14 patients (70%) had died (Table 1). During the retrospective evaluation, the 1-month postoperative survival rate was 90%, the 1-year survival rate was 84%, and the 3-year survival rate was 35%. The assessment of the 5-year survival rate was conducted among 13 cases who successfully completed the 5-year follow-up period, revealing a median OS of 35,0 months and a 5-year survival rate of 23%. All surviving patients were the ones who underwent left pneumonectomy. Of the patients who died, 7 were right pneumonectomy performed and 7 were left pneumonectomy performed patients. The mortality rate was 70% for all patients, 100.0% in patients with intrapericardial right pneumonectomy and 53.6% in patients with intrapericardial left pneumonectomy. The median OS value of the patients included in the study was found to be 22.3 months, 95% CI (5.6-39.0). There was no statistically significant difference between OS times in male and female patients (p = 0.233). The median OS of patients who received pre-operative CT/RT was 16.7 months, and the median OS of patients who did not receive pre-operative CT/RT was 47.9 months, and a borderline statistical difference was found between the two groups (p = 0.080). When median OS was evaluated according to histopathological diagnoses, it was observed that patients diagnosed with squamous cell carcinoma had the shortest median OS with 7.2 months. The median OS of patients who underwent intrapericardial right pneumonectomy was found to be statistically significantly shorter than that of patients who underwent intrapericardial left pneumonectomy (OS = 7.2 months, 95% CI (0.4-14.1) vs OS = 25.5 months 95% CI (4.5- 46.4) (p = 0.013) (Figure 1). Surgical margin status and post-operative RT/CT were found to have no effect on OS. In N2 cases, the median survival was 22.3 months 95 %CI (9.4-35.2), the 1-year survival rate was 71%, and the 3-year survival rate was 14%.
Surgical margin positivity was present in 4 cases. 3 of them were vascular surgical margin positivity, and one of these cases was accompanied by bronchial surgical margin positivity, one by mediastinal pleura, and one by pericardial surgical margin positivity. In one case, there was a positive bronchial surgical margin at the level of in situ carcinoma. All of them had microscopic surgical margin positivity and were accepted as R1. The median survival in these cases was calculated as 25.5 months.
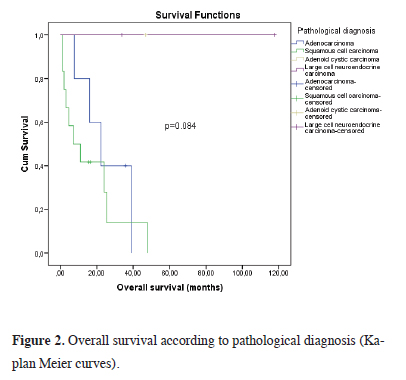
All patients who died before 1 year had developed additional pathologies such as pneumonia, bronchopleural fistula, empyema, and pulmonary thromboembolism. Additionally, these complications were detected only in patients who underwent right pneumonectomy. In 2 cases with bronchopleural fistula, the fistula was closed by placing a stent using rigid bronchoscopy. When all cases were evaluated, empyema with bronchopleural fistula developed in 2 cases, isolated empyema in 1 case, pneumonia in 2 cases, and vocal cord paralysis in 1 case in the postoperative period. Phonation was achieved by applying medialization thyroplasty to the patient who developed vocal cord paralysis during the hospitalization period. The mean OS was calculated as 16.8 months (1-48 months) in cases diagnosed with squamous cell carcinoma, 44 months (8-36 months) in cases diagnosed with adenocarcinoma, and 73 months (34-114 months) in cases with large cell neuroendocrine carcinomas. In the single case with adenoid cystic carcinoma, survival was achieved for 44 months (Figure 2).
 Click Here to Zoom |
Figure 1: Overall survival according to pneumonectomy side (Kaplan Meier curves). |
 Click Here to Zoom |
Figure 2: Overall survival according to pathological diagnosis (Kaplan Meier curves). |
Metastases developed in 25% of the patients. While four of them died, one was alive. Metastases were in various organs such as brain, vertebra, liver, opposite lung, kidneys, adrenals, and uterus. No recurrence or metastasis was observed in 15 patients (75.0%). Of the five patients who developed metastasis, 4 had undergone left pneumonectomy and 1 had undergone right pneumonectomy. All patients who developed recurrence had received adjuvant treatment, and lymphovascular space invasion and perinodal spread were observed in all patients. The median OS was 31.5 months for patients who developed metastasis and 36.0 months for patients who did not. There was no statistically significant difference (p = 0.598). Evaluation of factors affecting OS is presented in Table 2.
Table 2: Evaluation of factors affecting overall survival time (Kaplan Meier analysis).
According to the results of univariate COX regression analysis; histopathological diagnosis of squamous cell cancer increased the risk of mortality 3.3 times compared to other pathologies (HR = 3.3, 95% CI 1.1- 10.7) and p = 0.045). Intrapericardial pneumonectomy on the right side increased the risk of mortality 3.8 times (HR = 3.8, 95% CI (1.2-11.7) and p = 0.020). In addition, the risk of mortality was found to be higher in patients who received pre-operative CT than in those who did not, with borderline statistical significance (Table 3). In the multivariate COX regression analysis of the model created with these variables, the most important risk factors for mortality were right intrapericardial pneumonectomy (HR=4.3, 95% CI (1.2-15.7) and p = 0.029) and squamous cell cancer histopathology (HR= 7.1 95% CI (1.6-31.8) and p = 0.010) (Table 3).
Table 3: Evaluation of risk factors affecting mortality (COX regression analysis)
Discussion
Surgery, chemotherapy, radiotherapy, immunotherapy, and the combination of these treatment options with a multidisciplinary approach are used in the treatment of NSCLC. In NSCLC cases with pulmonary artery and pulmonary vein invasion, the possibility of surgical intervention persists despite local infiltration. An intrapericardial approach is required in some cases since it is essential to maintain safe surgical margins. In 1946, Allison et al. also argued that intrapericardial vascular ligation provides the opportunity for more radical surgery due to safer surgical margins in patients diagnosed with bronchial carcinoma and in whom pericardial invasion is observed [5]. Additionally, as the pericardium serves as a natural barrier, it has been argued that intrapericardial resection provides the opportunity to work in a safer area in terms of surgical margins, since it is difficult for the tumor to pass into the intrapericardial area through lymphatic drainage [6]. We also decided to perform pneumonectomy due to NSCLC in our clinic and preferred intrapericardial pneumonectomy in 20 cases with pulmonary artery and pulmonary vein between 2016 and 2020. In four of our cases, R1 resection with microinvasion was executed. Remarkably, within the series of 20 cases, R0 resection was achieved in 16 instances, underscoring the significant accomplishment despite the extensive surgical intervention.Although it is a good option for complete resection, pneumonectomy has a higher mortality and morbidity rate than other types of resections. The mortality rate after pneumonectomy varies between 3-20%, but it has been reported that right pneumonectomy has a higher mortality and morbidity rate than left pneumonectomy because the right lung is more hemodynamically effective than the left and is closer to fistula formation due to bronchial anatomy [2-6]. In addition, the anatomy of the right and left bronchial artery also shows various variations. However, the most common variation, with a rate of 60%, is the presence of one right bronchial artery and two left bronchial arteries [7]. This situation suggests that the blood supply of the right main bronchus may be weaker than the left and may be prone to fistula development.
In our cases, consistent with the literature, the mortality rate in patients who underwent right pneumonectomy was found to be higher and the average survival time to be shorter than in patients who underwent left pneumonectomy. It was revealed that patients who underwent right pneumonectomy had a higher HR for mortality. In addition, all our cases in which bronchopleural fistula, pulmonary thromboembolism, and empyema developed in the postoperative period consisted of patients with right intrapericardial pneumonectomy.
In addition to surgical treatment, discussing the necessity of neoadjuvant treatment with a multidisciplinary approach is imperative. While the mortality rate in patients receiving neoadjuvant treatment was found to be relatively lower, although not statistically significant, it has been shown that it is possible to survive for many years after pneumonectomy [8,9]. In our cases, mortality with a rate of 55% was observed among patients who did not undergo neoadjuvant treatment, while 44% of patients were alive, suggesting a potential beneficial impact of the implementation of neoadjuvant therapy on survival outcomes.
There are studies in the literature showing that squamous histopathological type is more aggressive than adeno cancers [10,11]. In our cases, the average overall survival time of patients with squamous histopathological type was found to be lower and their HR for mortality was higher
N2 involvement should be carefully evaluated in cases demonstrating localized invasion significant enough to require intrapericardial pneumonectomy. It has been stated in the literature that survival is generally worse, distant organ metastases are more common, and the recurrence rate is higher in cases of NSCLC and N2 [10,11]. The standard treatment in N2 cases is definitive chemoradiotherapy or induction therapy followed by surgery [12,13]. In the literature, N2 cases that underwent pneumonectomy after neoadjuvant treatment were defined as persistent N2 and it was stated that surgery had a positive effect on survival, but no significant difference was observed in survival in patients over the age of 65. Furthermore, immunotherapy has been reported to emerge as a component of multimodal therapy, with notable impact on survival outcomes in N2 cases. [14,15]. Among our cases, four out of the five patients who remain alive were classified as N1, with one being categorized as N0. Following neoadjuvant therapy, invasive staging identified five of the postoperative N2 cases as N0-N1, while 2 of the N2 cases were evaluated as N0-N1 by invasive staging in the preoperative period. Following a retrospective analysis of all postoperative N2 cases, the 1-year survival rate was determined to be 71%, while the 3-year survival rate stood at 14%.
In a series of 126 cases in which intrapericardial and standard pneumonectomy cases were compared in the literature, the average survival was calculated as 32 months in standard pneumonectomy and 23 months in intrapericardial pneumonectomy, and a significant difference was determined [16]. However, the median survival in our intrapericardial pneumonectomy cases was calculated as 22.3 months, and very good survival results were obtained compared to the cases in the literature that underwent intrapericardial pneumonectomy. With this article, we wanted to show that such aggressive surgeries can be beneficial in terms of gaining time for immunotherapy and other new generation treatments.
Limitations of the study
This study is a retrospective and single-center study,
the number of cases is limited even when the long-term
survival consequences of resection type are taken into account.
Studies with larger sample sizes will be more useful in detecting statistically significant differences. Third,
we could not compare patients who underwent pneumonectomy
with those who underwent sleeve resection and
arteroplasty in terms of recurrence and survival.
In conclusion, although conditions such as pulmonary artery, pulmonary vein or pericardial involvement negatively affect the disease prognosis in NSCLC, they are not considered as inoperability criteria. In these cases, which are local diseases, intrapericardial pneumonectomy can be performed to leave safe surgical margins. Mortality and morbidity are higher in right pneumonectomy than in left pneumonectomy. N2 disease is considered to have a poor prognosis.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect
to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research
and/or authorship of this article.
Ethics approval
This study was approved by Dokuz Eylül University
Non-invasive Research Ethics Committee with protocol
number 2022-CREC-22-41.
Authors’ contribution
BAS,AS; made substantial contributions to the design of
the work, FM,BKA; made the analysis of data, VK,BAS;
made the creation of new software used in the work,
OK,BAS; have drafted the work, AS,BAS: revised it. All
authors read and approved the final manuscript.
Reference
1) Licker M, Spiliopoulos A, Frey JG, Robert J, Höhn L, de Perrot
M et al. Risk factors for early mortality and major complications
following pneumonectomy for non-small cell carcinoma
of the lung. Chest 2002; 121: 1890-7.
2) Alpay L, Laçin T, Kıral H, Mısırlıoğlu A, Ocakcıoğlu I, Coşgun
T et al. Major Mortality and Morbidity Criteria in Pneumonectomies.
Maltepe Medical Journal 2013; 5: 17-23.
3) Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA
Cancer J Clin 2018; 68: 7-30
4) Subotic D, Savic M, Atanasijadis N, Gajic M, Stojsic J, Popovic
M et al. Standard versus extended pneumonectomy for lung
cancer: what really matters? World J Surg Oncol 2014; 12: 248.
5) Allison Pr. Intrapericardial approach to the lung root in the
treatment of bronchial carcinoma by dissection pneumonectomy.
J Thorac Surg 1946; 15: 99-117.
6) Oka S, Matsumiya H, Shinohara S, Kuwata T, Takenaka M,
Chikaishi Y et al. Intrapericardial Vessel Management for Lung
Cancer Surgery. J UOEH 2015; 37: 191-4.
7) Saluja S, Henderson KJ, White RI Jr. Embolotherapy in the
bronchial and pulmonary circulations. Radiol Clin North Am
2000; 38: 425-48.
8) Brunelli A, Rocco G, Szanto Z, Thomas P, Falcoz PE. Morbidity
and mortality of lobectomy or pneumonectomy after neoadjuvant
treatment: an analysis from the ESTS database. Eur J
Cardiothorac Surg 2020; 57: 740-6.
9) Martin J, Ginsberg RJ, Abolhoda A, Bains MS, Downey RJ,
Korst RJ et al. Morbidity and mortality after neoadjuvant therapy
for lung cancer: the risks of right pneumonectomy. Ann
Thorac Surg 2001; 72: 1149-54.
10) Wen CT, Fu JY, Wu CF, Liu YH, Wu CY et al. Risk factors for
relapse of resectable pathologic N2 non-small lung cancer and prediction
model for time-to-progression. Biomed J 2017; 40: 55-61.
11) Vansteenkiste JF, De Leyn PR, Deneffe GJ, Stalpaert G, Nackaerts
KL, Lerut TE et al. Survival and prognostic factors in resected
N2 non-small cell lung cancer: a study of 140 cases. Leuven
Lung Cancer Group. Ann Thorac Surg 1997; 63: 1441-50.
12) Rosell R, Gómez-Codina J, Camps C, Maestre J, Padille J,
Cantó A et al. A randomized trial comparing preoperative chemotherapy
plus surgery with surgery alone in patients with
non-small-cell lung cancer. N Engl J Med 1994; 330: 153-8.
13) Pless M, Stupp R, Ris HB, Stahel RA, Weder W, Thierstein S
et al. SAKK Lung Cancer Project Group. Induction chemoradiation
in stage IIIA/N2 non-small-cell lung cancer: a phase 3
randomised trial. Lancet 2015; 386: 1049-56.
14) Shah AA, Worni M, Kelsey CR, Onaitis MW, D’Amico TA,
Berry MF. Does pneumonectomy have a role in the treatment
of stage IIIA non-small cell lung cancer? Ann Thorac Surg
2013; 95: 1700-7.






