2Department of Thoracic Surgery, Health Sciences University, Sureyyapasa Chest Diseases and Thoracic Surgery Training and Research Hospital, Istanbul, Turkey
3Department of Thoracic Surgery Memorial Hospital, Istanbul, Turkey DOI : 10.26663/cts.2025.009
Summary
Background: Although stage IV non-small cell lung cancer (NSCLC) is often considered incurable, a growing body of evidence suggests that patients with oligometastatic disease-characterized by a limited number of metastases-may benefit from aggressive local treatment. This study evaluates the outcomes of patients with synchronous extrathoracic oligometastases and explores factors influencing survival.Materials and Methods: We retrospectively analyzed 35 consecutive patients (4 females, 31 males) diagnosed with NSCLC who presented with synchronous extrathoracic metastases. All patients underwent standardized staging, including cervical mediastinoscopy, and, where feasible, both the primary tumor and metastatic lesions were managed with curative intent (surgery or ablative therapies).
Results: Overall mean survival was 38 ± 6 months; when hospital mortality (n = 4) was excluded, mean survival rose to 43 ± 7 months with a median of 27 months. The 1-, 2-, and 5-year survival rates were 58.9%, 47.7%, and 38.1%, respectively. Brain metastases were the most frequent site (74% of patients), and patients with brain metastases insignificantly exhibited higher mortality compared to other metastasis sites. Neither age, smoking history, nor tumor laterality significantly affected survival, and histological subtype did not confer a clear survival advantage.
Conclusions: In selected patients with synchronous oligometastatic NSCLC, aggressive treatment targeting both the primary tumor and metastases can extend survival beyond historical expectations for stage IV disease. Further large-scale prospective studies are needed to identify the subgroups most likely to benefit from bifocal (primary and metastatic) surgical management.
Introduction
Lung cancer is the most common cause of cancer-related death worldwide. In 2020, there were approximately 1.8 million deaths due to lung cancer [1]. Non-small cell lung cancer (NSCLC) accounts for about 85% of all lung cancers, and a significant portion of these patients are in advanced stage at the time of diagnosis. Especially in metastatic (Stage IV) NSCLC, the prognosis is poor; modern series report a 5-year relative survival rate of only ~9% in patients with metastatic lung cancer (Non-Small Cell Lung Cancer Treatment (PDQ®) - NCI). Nonetheless, it has become clear that Stage IV disease is a heterogeneous group, and a subgroup with a limited number of metastases has been identified. In the condition termed “oligometastatic,” the number and distribution of distant metastases are limited, and longterm survival can be achieved in suitable patients with aggressive local therapies [2].In oligometastatic NSCLC, the most commonly involved organs are the brain and adrenal gland, followed by bone and other sites [3]. In particular, in the presence of synchronous (simultaneous) solitary brain or adrenal metastasis, administering curative treatments for both the primary lung tumor and the metastasis in eligible patients has historically yielded promising results. For example, in NSCLC cases with a single brain metastasis, surgical resection of the brain metastasis or stereotactic radiosurgery combined with lung resection can achieve long-term survival [4]. Similarly, various studies have reported 5-year survival rates in the range of 25–30% in cases where lung surgery was performed together with metastasectomy for adrenal gland metastasis. These data indicate the potential curative role of aggressive local therapies in oligometastatic NSCLC [5-7].
Current guidelines and studies support a multidisciplinary, individualized approach in patients with oligometastatic NSCLC. The European Society for Medical Oncology (ESMO) guideline recommends adding local ablative therapies to systemic treatment in appropriate patients and emphasizes that surgical or stereotactic radiotherapy for oligometastatic foci should be considered regardless of molecular profile [3]. The National Comprehensive Cancer Network (NCCN) guidelines similarly recommend administering definitive/curative treatments for the metastatic focus(es) and the primary tumor. In particular, in the case of a solitary synchronous brain metastasis, it is recommended to apply stereotactic radiosurgery or neurosurgery to the metastasis first, followed by definitive chest treatment (surgical resection or radiotherapy) [4]. This approach aims to increase the chance of long-term disease control by treating the primary tumor after controlling the metastasis. Phase II randomized studies conducted in recent years have also shown that adding local consolidative therapy (via surgery or radiotherapy) to systemic treatment can improve both progression-free survival and overall survival [4,8,9]. In light of all these data, surgical treatment emerges as an important option in appropriate patients with limited metastatic NSCLC. In this study, we evaluated the outcomes of surgical treatments applied to patients with synchronous solitary extrathoracic oligometastatic NSCLC. To this end, we examined the demographic and tumor-related characteristics of patients in our clinic who underwent surgery for both primary lung cancer and the metastatic lesion, the treatments applied, survival and recurrence outcomes, and analyzed prognostic factors and the effects of surgical approaches.
Methods
Study design and patient selectionIn this retrospective cohort study, we examined NSCLC patients treated in our clinic between January 2012 and December 2017 who presented with a synchronous single extrathoracic metastasis at the time of diagnosis. The inclusion criteria for the study were: (1) a histologically confirmed diagnosis of NSCLC, (2) the presence of a solid extrathoracic organ metastasis, and (3) the application of curative-intent treatment via anatomical lung resection (lobectomy or pneumonectomy) and metastasis-focused therapy. The distant metastasis was considered synchronous, meaning it was diagnosed simultaneously with the primary lung cancer. Cases deemed inoperable for surgical treatment or those in which anatomical resection was not feasible were excluded from the study. A total of 35 consecutive patients who met these criteria during the specified period were included. All patients provided informed consent prior to treatment. The study protocol was reviewed and approved by the Institutional Review Board of Istanbul Aydın University (Approval No. 102/2024). The research was conducted in accordance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects. Given the retrospective nature of the study, all patient data were anonymized prior to analysis.
Diagnosis and staging
Standard staging protocols were applied in patients’ preoperative
evaluations. All cases underwent contrast-enhanced
thoracic computed tomography (CT), 18F-FDG
positron emission tomography/computed tomography
(PET/CT), and cranial magnetic resonance imaging (MRI) to assess the primary tumor and metastatic foci.
For patients in whom PET/CT showed suspicious extrathoracic
lesions, such as in the abdomen, advanced
imaging (e.g., abdominal MRI) was performed. To confirm
the diagnosis, each patient underwent endobronchial
biopsy via fiber-optic bronchoscopy; in suitable
cases with peripheral tumors, samples were also taken
by transthoracic fine needle aspiration biopsy (TTFNA),
and histopathological diagnoses were confirmed. As
part of preoperative staging for the primary lung tumor,
cervical mediastinoscopy was performed in all cases to
exclude mediastinal lymph node metastasis. Patients
for whom mediastinoscopic biopsy results revealed no
N2/N3 metastatic lymph node involvement were found
suitable for surgical resection.
Surgical treatment and perioperative management
Treatment strategies were determined in a multidisciplinary
manner depending on the location of the metastasis.
In patients with brain metastases, metastasis-directed
treatment was performed first, guided by the size, number,
and location of the metastasis: either neurosurgery
or stereotactic radiosurgery (SBRT) [4]. The metastasis
management in patients with brain metastases was personalized
through joint decisions by neurosurgery and
radiation oncology specialists. About three to four weeks
(on average 24 days, range 17-48 days) following successful
treatment of the brain metastasis, anatomical resection
of the primary lung cancer was carried out. For
patients with metastases in sites outside the brain - adrenal
gland, spleen, or ocular region - the process was
reversed: first, the primary lung lesion was resected, then,
after a brief recovery period, the metastatic lesion was
surgically excised. Metastasectomy was the preferred
treatment modality, and in all cases with non-cerebral
solitary metastases, the metastatic lesion was removed
surgically. All thoracic surgeries were performed under
general anesthesia via a posterolateral thoracotomy, during
which anatomical resections (lobectomy or pneumonectomy)
were performed. Systematic mediastinal
lymph node dissection was carried out during lung resection.
Postoperative care was managed according to standard
protocols, and all patients were referred to medical
oncology in the postoperative period to receive necessary
oncological treatments, such as adjuvant chemotherapy
and/or radiotherapy. Deaths occurring within 30 days of
the operation were defined as “hospital mortality.”
Statistical Analysis
The demographic characteristics of the patients (age,
sex), smoking history (pack-years), localization of the
primary tumor (right/left and specific lobes), tumor size and histological type, metastasis site and the therapy used
for metastasis, type of lung operation performed, hospital
length of stay post-surgery, postoperative complications,
and mortality were all recorded. In pathological staging,
the pT and pN stages were determined in accordance
with the 8th edition of the TNM classification [10]. The
patients were followed for the development of recurrence
or new metastases, as well as for any treatments administered
in such cases (e.g., salvage treatments in the event
of recurrence). The data obtained were analyzed using
the IBM SPSS or NCSS 2007 (Kaysville, Utah, USA)
software package. Continuous variables were reported as
mean ± standard deviation or median (minimum–maximum).
Categorical variables were summarized as numbers
and percentages. Appropriate statistical tests were
used for comparisons; for instance, normally distributed
quantitative data were evaluated between two groups
using Student’s t-test. Fisher’s exact test or the Fisher-
Freeman-Halton test was used for comparing categorical
variables. Survival analyses were performed via the Kaplan-
Meier method, and comparisons of survival curves
across groups were carried out using the Log-rank test.
Overall survival was defined as the time from the date of
thoracic surgery to the date of death or the last follow-up.
A p-value of <0.05 was deemed statistically significant.
Results
Among the 35 patients included in the study, 31 were men and 4 were women (male ratio of 88.6%). At the time of diagnosis, patients ranged in age from 39 to 77 years, with a mean age of 60.1 ± 8.3 years. Just over half of the cases (n = 18, 51.4%) were 60 years of age or younger, and the remaining 48.6% were older than 61. The majority of the patients were smokers, with an average consumption of around 30 pack-years; 60% had a smoking history of ≥20 pack-years (Table 1). Regarding the location of the primary lung tumors, 37.1% were in the right upper lobe, 31.4% in the left upper lobe, 25.7% in the left lower lobe, and 5.7% in the right lower lobe. Histopathological examination revealed that 71.4% (n = 25) of the cases were adenocarcinoma, 25.7% (n = 9) were squamous cell carcinoma, and 2.9% (n = 1) were large cell carcinoma. Distribution of the pathological T stage was most frequently T2 (57.1%, n = 20), followed by T3 (28.6%, n = 10). Three patients (8.6%) were staged as T1, and two (5.7%) were T4. In terms of pathological N stage, nearly half of the patients had no mediastinal lymph node metastasis: 54.3% (n = 19) were N0, 25.7% (n = 9) were N1, and 20% (n = 7) were N2. All patients were clinically classified as Stage IVA (M1b) due to the presence of a single distant metastasis.Table 1: Survival analysis by age (n=31).
Metastasis and treatment characteristics
The most frequently observed metastasis site was the
brain; 26 of the 35 patients (74.3%) had synchronous
brain metastases. Adrenal gland metastases were found
in 6 patients (17.1%). Three patients (8.6%) had metastases
in other organs (2 in the spleen, 1 in the eye).
Curative-intent treatment was carried out in all cases for
metastatic foci: in 80.0% (n = 28), the metastasis was
removed surgically (metastasectomy), while in 20.0%
(n = 7) it was managed with stereotactic radiosurgery
(Table 2). Radiosurgery was applied only in patients
with brain metastases, and in a subset of these, SBRT
was chosen instead of neurosurgery. Examination of the
surgical resections for the primary lung tumor showed
that 29 patients underwent lobectomy (82.9%) and 6
underwent pneumonectomy (17.1%). Postoperative
hospital stays ranged from 3 to 12 days, with a median
of 5 days (mean 5.9 ± 2.3 days) (Table 2). The number
of patients who developed major postoperative complications
was small, and four patients died within 30 days
of surgery (categorized as “hospital mortality”).
Table 2: Survival Analysis by metastasis (n=31).
Oncological results
Following the completion of appropriate adjuvant treatments
determined by pathological stage, patients were
followed up on a regular basis. The median follow-up
time was 14 months (range: 0-81 months). Within this
period, 4 patients (11.4%) experienced disease recurrence
(local recurrence and/or new distant metastases);
in most patients (88.6%), the disease remained under
control during follow-up. At the last follow-up, 17 patients
(48.6%) were alive, while 18 (51.4%) had died.
Since 4 of those 18 died within the first month postoperatively,
the perioperative mortality rate was calculated
as 11.4%. All four 30-day deaths occurred in patients
with synchronous brain metastases. In case 1, postoperative
day 3 – sepsis progressing to multi-organ failure
after cranial metastasectomy + left upper lobectomy. In
case 2, postoperative day 10 – pneumonia leading to
respiratory failure after stereotactic body radiotherapy
(SBRT) to the brain lesion + right pneumonectomy. In
case, postoperative day 22 – acute respiratory failure following SBRT + right upper lobectomy. In case 4,
postoperative day 25 - acute myocardial infarction after
SBRT + sleeve right upper lobectomy. Excluding these
early deaths (i.e., censored), the overall survival analysis
(n = 31) showed a median survival of 27 months (Table
3). The 1 and 2- year survival rates were 59% and 48%,
respectively, while the 5-year survival rate was about
38%. Examination of the Kaplan-Meier curve indicated
that the last death occurred in the 38th month and that the
survival rate stabilized at around 39% at about 3 years.
Prognostic analyses
Survival by site of metastasis is summarized in Table 2,
where 22 patients had brain metastases and 6 had adrenal
metastases. In the brain-metastasis group, 10 patients
(45.5%) survived, 12 died, and the mean survival was
26 ± 4 months (median: 20 months). Among patients
with adrenal metastases, all 6 were alive (100%) at their
last follow-up, yielding a mean survival time of 21 ±
17 months (median: 18 months). Because no deaths occurred
in the adrenal group, statistical comparison among
metastasis sites was not feasible. The survival curve by
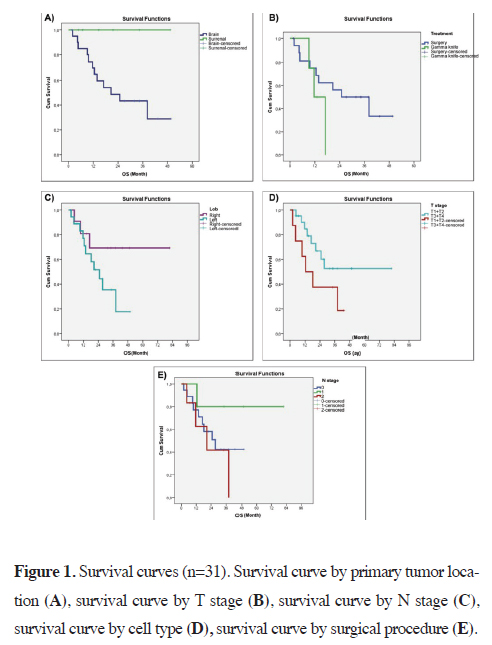
metastasis site is displayed in Figure 1A.
 Click Here to Zoom |
Figure 1: Survival curves (n=31). Survival curve by primary tumor location (A), survival curve by T stage (B), survival curve by N stage (C), survival curve by cell type (D), survival curve by surgical procedure (E). |
Table 2: Survival Analysis by metastasis (n=31).
Further analysis in patients with brain metastases appears in Table 3, which contrasts survival in those who underwent cranial surgery versus radiosurgery. Of the 18 who had cranial surgery, 9 (50.0%) survived, and the mean survival was 28 ± 4 months (median: 24 months). In the 4 who received radiosurgery, 1 (25.0%) remained alive, and the mean survival was 13 ± 2 months (median: 11 months). A Log Rank test found no statistically significant difference between these two treatment subgroups (p > 0.05). These results appear in Figure 1B. In terms of primary tumor location, Table 4 shows that 12 patients had right-lung tumors (9 alive, 3 deceased) and 19 had left-lung tumors (8 alive, 11 deceased). The mean survival was 59 ± 10 months for right-sided tumors (median: 24 months) and 25 ± 4 months for left-sided tumors (median: 24 months). The difference by tumor laterality was not significant (p > 0.05), and their survival curves are provided in Figure 1C. When stratified by T stage, as indicated in Table 5, 22 patients were in T1+T2 (14 alive, 8 deceased) with a mean survival of 51 ± 8 months (median: 21 months), whereas 9 were in T3+T4 (3 alive, 6 deceased) with a mean survival of 21 ± 5 months (median: 12 months). Although survival was numerically longer in T1+T2, the Log Rank test did not show a statistically significant difference (p = 0.125; p > 0.05). The T-stage survival curves can be seen in Figure 1D. Survival by N stage is presented in Table 6: N0 included 18 patients (9 alive, 9 deceased) with a mean survival of 29 ± 4 months (median: 27 months), N1 included 6 patients (5 alive, 1 deceased) with a mean survival of 67 ± 12 months (median: 23 months), and N2 included 7 patients (3 alive, 4 deceased) with a mean survival of 23 ± 6 months (median: 20 months). No statistically significant difference emerged among N0, N1, and N2 (p > 0.05). The survival curves by N stage are shown in Figure 2. With regard to cell type, Table 7 indicates that, among 21 patients diagnosed with adenocarcinoma, 12 (57.1%) remained alive (mean survival: 46 ± 8 months, median: 27 months), while among 9 diagnosed with squamous cell carcinoma, 5 (55.6%) were alive (mean survival: 28 ± 5 months, median: 38 months). Large cell carcinoma was excluded from the comparative analysis because it was observed in only one patient. No statistically significant difference emerged between adenocarcinoma and squamous cell carcinoma (p > 0.05).
Table 3: Survival analysis by treatment type in patients with brain metastases (n=22).
Table 4: Survival analysis by primary tumor location.
Table 5: Survival analysis by T stage (n=31).
Table 6: Survival analysis by N stage (n=31).
Table 7: Survival analysis by cell type (n=31).
Regarding surgical procedure, Table 8 shows that 26 patients underwent lobectomy (16 alive, 10 deceased) and 5 underwent pneumonectomy (1 alive, 4 deceased). Mean survival was 49 ± 7 months (median: 18 months) in the lobectomy group and 23 ± 6 months (median: 27 months) in the pneumonectomy group. Although pneumonectomy had fewer survivors numerically, the Log Rank test did not indicate a statistically significant difference (p > 0.05). Lastly, to explore risk factors potentially affecting mortality, a logistic regression analysis was conducted, as summarized in Table 9. Metastasis, advanced T stage, and surgery type were included because their effects approached significance in earlier steps. The overall model was significant and explained around 64.5% of variance in mortality. However, none of these variables-metastasis, T stage, or surgeryreached statistical significance (p > 0.05).
Table 8: Survival analysis by surgical procedure (n=31).
Table 9: Logistic regression analysis of risk factors for mortality.
Discussion
Although the current NCCN guideline provides no specific treatment recommendations for other extrathoracic metastases, it does recommend aggressive local therapy for NSCLC with oligometastatic disease in the brain or adrenal gland [11]. In our clinic, all lung cancer patients routinely undergo thoracic CT, PET/CT, and cranial MRI for metastasis screening. In the event that suspicious extrathoracic lesions are detected on imaging, further imaging methods targeting those lesions are used. MRI was performed for six patients with adrenal metastases, two with splenic metastases, and one with an ocular metastasis when metastasis was suspected. We included 35 consecutive patients (4 women, 31 men) diagnosed with lung cancer who had synchronous extrathoracic metastases. In our study, patient follow-up ranged from 0 to 81 months, with a mean of 20 months. The mean survival time was 38 ± 6 months. After censoring hospital mortality (n = 4), the remaining 31 patients had a mean survival of 43 ± 7 months and a median survival of 27 months. The 1, 2, and 5-year survival rates were 58.9%, 47.7%, and 38.1%, respectively. In the phase II study by De Ruysscher et al with 40 patients having synchronous oligometastatic NSCLC, the median survival was 13 months, and the 1-, 2-, and 3-year survival rates were 56.4%, 23.3%, and 17.5%, respectively [12]. In the series by Plönes et al, which included 56 cases of synchronous oligometastatic NSCLC, overall survival was 14 months [13]. In the 29-case series of oligometastatic NSCLC by Collaud et al, the 5-year survival rate was 36.8%, and the median survival was 20 months [14]. In the 75-case series published by Fleckenstein et al, which included both synchronous and metachronous metastases, the median follow-up was 54 months; the median survival was 21 months; and the 1-, 2-, and 5-year survival rates were 78%, 44%, and 27%, respectively [15]. In a review by Ashworth et al involving 2176 cases, the median survival was 14 months, while the 1-, 2-, and 5-year survival rates were 57.2%, 42%, and 23.3%, respectively [16]. Over the course of follow-up in our study, 51.4% (n = 18) of patients died, while 48.6% (n = 17) remained alive. Among those who died, the hospital mortality rate was 11.4% (n = 4). Our in-hospital mortality rate was found to be higher than in the literature. All four patients who experienced hospital mortality had brain metastases. One patient with adenocarcinoma, who underwent cranial metastasectomy followed by mediastinoscopy and left upper lobectomy, died on postoperative day 3 from sepsis and multi-organ failure. Another patient with adenocarcinoma, who underwent stereotactic body radiotherapy (SBRT) followed by mediastinoscopy and right pneumonectomy, died of pneumonia and respiratory failure on postoperative day 10. A third patient, diagnosed with adenocarcinoma, who underwent SBRT followed by mediastinoscopy and right upper lobectomy, died from acute respiratory failure on postoperative day 22. Finally, a patient diagnosed with adenocarcinoma, who underwent SBRT followed by mediastinoscopy and a sleeve right upper lobectomy, died of acute myocardial infarction on postoperative day 25. Although reported hospital mortality after lung resection is typically lower, we believe that an 11.4% in-hospital mortality rate is acceptable for patients with advanced-stage disease. In our series, postoperative complications related to surgery included pneumonia in two patients, supraventricular tachycardia in two patients, and wound infection in one patient. All five patients who developed postoperative complications recovered with medical treatment.In our series, patient ages ranged from 39 to 77, with an average of 60 ± 8 years. In our study, 55.6% (n = 18) of patients were 60 years old or younger, while 48.6% (n = 17) were 61 or older. We found no relationship between age and survival (p = 0.461). After censoring hospital mortality, mean survival for patients aged ≤60 years (n = 17) was 36 ± 8 months with a median survival of 20 months, whereas for those ≥61 (n = 14), the mean survival was 34 ± 4 months. In a series by Pessini et al, survival was poorer among those over 70 [17]. In a review by Ashworth et al of 2176 oligometastatic NSCLC cases and a study by Bai et al involving 76 patients with synchronous brain oligometastases, age was reported as a poor prognostic factor for overall survival. In our study, no statistically significant difference in survival was found between these age groups (p > 0.05). Among our patients, 40.0% (n = 14) had a smoking history of <20 pack-years, 31.4% (n = 11) had 21–39 pack-years, and 28.6% (n = 10) had >40 packyears. In the series by Guerra et al, which included 78 oligometastatic NSCLC patients treated with definitive chemoradiotherapy, heavy smoking was found to be a poor prognostic factor. In the study by Bai et al, smoking had a negative effect on survival [18]. In our series, smoking history had no impact on survival (p = 0.360).
In the series by Barone et al and Johnson et al, no effect on survival was observed when the side (laterality) of the surgery was evaluated. In reviews by Novoa et al and Ashworth et al, lobectomy was identified as a better prognostic factor for survival [19]. In the study by Plönes et al, the type of lung resection showed no relationship with survival [20]. In our study, 5.7% (n = 2) of primary tumors were located in the right lower lobe, 37.1% (n = 13) in the right upper lobe, 25.7% (n = 9) in the left lower lobe, and 31.4% (n = 11) in the left upper lobe, with no significant effect of tumor laterality on survival (p = 0.456). After censoring hospital mortality, the mean survival was 59 ± 10 months (median: 24) for right-sided tumors and 25 ± 4 months (median: 24) for left-sided tumors. No significant survival difference was found based on tumor side (p > 0.05). In evaluating tumor histology, we found that 71.4% (n = 25) of patients had adenocarcinoma, 25.0% (n = 9) had squamous cell carcinoma, and 3.6% (n = 1) had large cell carcinoma. Consistent with the literature, adenocarcinoma was the most frequently observed histological type. In the series by Plönes et al, median survival according to cell type was 18 months for adenocarcinoma, 14 months for squamous cell carcinoma, and 8 months for large cell carcinoma; no statistically significant difference was found based on histological subtype. After censoring hospital mortality in our study, for adenocarcinoma, the mean survival time was 46 ± 8 months (median: 27), and for squamous cell carcinoma, it was 28 ± 5 months (median: 38). Since only one patient had large cell carcinoma, no separate survival analysis was performed for that subgroup. In reviews by Novoa et al. and Ashworth et al, adenocarcinoma was reported as a favorable prognostic factor [21]. However, in the series by Fleckenstein et al and Collaud et al, no difference in prognosis was found between adenocarcinoma and other histological types. In our series as well, when comparing histological subtypes, no statistically significant difference in survival was observed (p > 0.05). For an accurate evaluation of the N (nodal) stage in oligometastatic disease, invasive mediastinal staging is recommended. In our study, all patients underwent cervical mediastinoscopy to evaluate mediastinal lymph node status, and among those confirmed pathologically as N0, 29 underwent lobectomy and 6 underwent pneumonectomy. In the literature, better survival has been reported in patients undergoing lobectomy [21]. After censoring hospital mortality, the mean survival was 49± 7 months (median: 18) in patients who underwent lobectomy and 23 ± 6 months (median: 27) in those who underwent pneumonectomy. We found no statistically significant difference in survival between types of surgical procedures (p > 0.05).
In patients with synchronous brain metastases, the treatment strategy should address the metastasis first. Metastasis therapy options include cranial surgery, radiosurgery, WBRT, or post-surgical radiosurgery [17]. While guidelines provide no definitive recommendation for curative resection, they do suggest WBRT following a curative resection [22]. In our series, of 26 patients with synchronous isolated brain metastases, 26.9% (n = 7) underwent SBRT and 73.1% (n = 19) underwent cranial surgery with metastasectomy. Following metastasis treatment, pneumonectomy was performed in 5 patients and lobectomy in 21. There was no statistical difference regarding treatments of the metastasis and primary tumor. Postoperatively, 13 patients received adjuvant chemotherapy (CT) alone, 3 had WBRT, 6 had WBRT plus CT and thoracic RT, and 4 had WBRT plus CT. Recurrent lesions were identified in four patients, who underwent additional surgical resection. After censoring hospital mortality, survival analysis showed that the mean survival was 13 ± 2 months (median: 11) in those who had SBRT for metastases and 28 ± 4 months (median: 24) in those who underwent cranial surgery. There was no statistically significant difference in survival based on the type of metastasis treatment (p > 0.05). Among 26 patients with brain metastases, the mean survival time was 26 ± 4 months (median: 20), with 1-, 2-, and 5-year survival rates of 50.2%, 43.0%, and 28.7%, respectively, when hospital mortality was censored. In NSCLC patients with adrenal metastases, a five-year survival rate of 25% has been reported if both the primary tumor and adrenal metastasis can be resected. The literature indicates that adrenal metastases occur in 1.5–3.5% of NSCLC cases. Tantevyanon et al reported in a review of 114 patients with synchronous adrenal metastases that overall survival was 12 months and the five-year survival was 26% [5]. In a study of 37 patients by Raz et al, adrenalectomy, ipsilateral metastasis, N0/1 status, and synchronous metastasis were cited as favorable prognostic factors for patients with adrenal metastases [7]. In a 37-patient series published by Barone et al, the five-year median survival rate for those undergoing adrenalectomy was 29.3% [23]. Treatment options for adrenal metastases include surgery or radiosurgery; however, surgical intervention offers better survival outcomes [23]. In patients with isolated adrenal metastases in NSCLC who undergo complete resection of the lung tumor and have no other metastases, surgery or radiosurgery is recommended. In our series, after resection of the primary tumor, six patients underwent a surgical adrenalectomy. Their mean overall survival was 21 ± 17 months, with a median of 18 months. Postoperatively, five patients received adjuvant CT, and one received adjuvant CT plus thoracic RT.
In a review by Salah et al involving 75 oligometastatic cases (excluding brain and adrenal metastases), a five-year survival rate of 50% was reported. In that series, which was predominantly composed of adenocarcinoma cases undergoing lobectomy, nodal involvement was found to be the main factor influencing survival [22]. In autopsy studies of cancer patients, the prevalence of splenic metastasis has been reported to be 2-7%. In our series, of two patients with splenic metastases, one underwent simultaneous splenectomy with lung resection, while the other had a post-resection splenectomy. Both received adjuvant CT postoperatively. Although current guidelines do not recommend metastasectomy for lesions beyond the brain and adrenal glands, the survival times for these two patients were 18 months for the first and 81 months for the second. About 30% of ocular metastases originate from lung cancer. The incidence of choroidal metastases in lung carcinoma is 2-6.7% [24]. Treatment options for choroidal metastases include radiotherapy, plaque radiotherapy, surgical resection, transpupillary thermotherapy, and intravitreal chemotherapy [25]. Survival ranges from 5 to 19 months in patients with choroidal metastases secondary to a lung primary [25]. In our series, the patient with ocular metastasis underwent surgical enucleation and received adjuvant chemotherapy in the postoperative period; that patient died in the 27th month after pulmonary resection. In earlier years, stage IV disease was managed conservatively, and survival was limited to 8-11 months. However, with recent advances in surgery and staging, as well as large published series, survival has improved in patients with oligometastatic disease. In selected oligometastatic patients, complete resection of the primary tumor and curative treatment of metastases can prolong survival. Although our patients with brain metastases had poorer survival compared to patients with other metastases, our data suggest that aggressive therapy improves survival in oligometastatic NSCLC. Our series, as well as an increasing number of studies in the literature, demonstrate prolonged survival in patients undergoing bifocal surgical treatment.
Declaration of conflicting interests
The authors declared no conflict of interest with respect
to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research
and/or authorship of this article.
Ethics approval
This study was approved by the Istanbul Aydin University
Non-invasive Research Ethics Committee with protocol
number 102/2024, and conducted in accordance
with the Declaration of Helsinki.
Authors’ contribution
IK,HK, Design: IK,HK,CT, Definition of intellectual
content: RSE,VB, Literature Search: IK,EEK, Clinical
Studies:IK,EEK,RSE,HK,CT,VB, Data acquisition:
IK,HK, Statistical analysis: IK, Manuscript preparation:
IK, Editing: HK,VB, Review:RSE,CT
Reference
1) Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I,
Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN
Estimates of Incidence and Mortality Worldwide for 36 Cancers
in 185 Countries. CA Cancer J Clin 2021; 71: 209-249.
2) Euhus CJ, Ripley TR, Medina CG. The Role of Surgery for
Oligometastatic Non-Small Cell Lung Cancer. Cancers (Basel)
2022; 14: 2524.
3) Torresan S, Costa J, Zanchetta C, De Marchi L, Rizzato S, Cortiula
F. Oligometastatic NSCLC: Current Perspectives and Future
Challenges. Curr Oncol 2025; 32: 75.
4) Kumar A, Kumar S, Potter AL, Raman V, Kozono DE, Lanuti
M, Jeffrey Yang CF. Surgical management of non-small cell
lung cancer with limited metastatic disease involving only the
brain. J Thorac Cardiovasc Surg 2024; 167: 466-477.
5) Tanvetyanon T, Robinson LA, Schell MJ, Strong VE, Kapoor
R, Coit DG, Bepler G. Outcomes of adrenalectomy for isolated
synchronous versus metachronous adrenal metastases in nonsmall-
cell lung cancer: a systematic review and pooled analysis.
J Clin Oncol 2008; 26: 1142-7.
6) Gao XL, Zhang KW, Tang MB, Zhang KJ, Fang LN, Liu W.
Pooled analysis for surgical treatment for isolated adrenal metastasis
and non-small cell lung cancer. Interact Cardiovasc
Thorac Surg 2017; 24: 1-7.
7) Raz DJ, Lanuti M, Gaissert HC, Wright CD, Mathisen DJ,
Wain JC. Outcomes of patients with isolated adrenal metastasis
from non-small cell lung carcinoma. Ann Thorac Surg 2011;
92: 1788-92; discussion 1793.
8) Varela G, Thomas PA. Surgical management of advanced nonsmall
cell lung cancer. J Thorac Dis 2014; 6 Suppl 2: S217-23.
9) Deboever N, Mitchell KG, Feldman HA, Cascone T, Sepesi B.
Current Surgical Indications for Non-Small-Cell Lung Cancer.
Cancers (Basel) 2022; 14: 1263.
10) O’Sullivan B, Asamura H, Lee A, Van Eycken E, Denny M,
Amin M. TNM Classification of Malignant Tumours. Head and
Neck Tumours. 8th ed. John Wiley & Sons, Ltd, 2017: p. 18-21.
11) Iyengar P, All S, Berry MF, Boike TP, Bradfield L, Dingemans
AC, et al., Treatment of Oligometastatic Non-Small Cell Lung
Cancer: An ASTRO/ESTRO Clinical Practice Guideline. Pract
Radiat Oncol 2023; 13: 393-412.
12) Dingemans AC, Hendriks LEL, Berghmans T, Levy A, Hasan
B, Faivre-Finn C et al., Definition of Synchronous Oligometastatic
Non-Small Cell Lung Cancer-A Consensus Report. J
Thorac Oncol 2019; 14: 2109-19.
13) George J, Maas L, Abedpour N, Cartolano M, Kaiser L, Fischer
RN et al., Evolutionary trajectories of small cell lung cancer
under therapy. Nature 2024; 627: 880-9.
14) Collaud S, Stahel R, Inci I, Hillinger S, Schneiter D, Kestenholz
P, Weder W. Survival of patients treated surgically for
synchronous single-organ metastatic NSCLC and advanced
pathologic TN stage. Lung Cancer 2012; 78: 234-8.
15) Stelljes M, Krug U, Beelen DW, Braess J, Sauerland MC, Heinecke
A et al., Allogeneic transplantation versus chemotherapy
as postremission therapy for acute myeloid leukemia: a prospective
matched pairs analysis. J Clin Oncol 2014; 32: 288-96.
16) Ashworth AB, Senan S, Palma DA, Riquet M, Ahn YC, Ricardi
U et al., An individual patient data metaanalysis of outcomes
and prognostic factors after treatment of oligometastatic nonsmall-
cell lung cancer. Clin Lung Cancer 2014; 15: 346-55.
17) Pessina F, Navarria P, Cozzi L, Ascolese AM, Maggi G, Riva M
et al., Outcome Evaluation of Oligometastatic Patients Treated
with Surgical Resection Followed by Hypofractionated Stereotactic
Radiosurgery (HSRS) on the Tumor Bed, for Single,
Large Brain Metastases. PLoS One 2016; 11: e0157869.
18) Bai H, Xu J, Yang H, Jin B, Lou Y, Wu D, Han B. Survival
prognostic factors for patients with synchronous brain oligometastatic
non-small-cell lung carcinoma receiving local therapy.
Onco Targets Ther 2016; 9: 4207-13.
19) Lanuti M. Surgical management of oligometastatic non–small
cell lung cancer. Thorac Surg Clin 2016: 26: 287-94.
20) Plönes T, Osei-Agyemang T, Krohn A, Passlick B. Surgical
Treatment of Extrapulmonary Oligometastatic Non-small Cell
Lung Cancer. Indian J Surg 2015; 77(Suppl 2): 216-20.
21) Novoa NM, Varela G, Jiménez MF. Surgical management of
oligometastatic non-small cell lung cancer. J Thorac Dis 2016;
8(Suppl 11): S895-S900.
22) Salah S, Tanvetyanon T, Abbasi S. Metastatectomy for extracranial
extra-adrenal non-small cell lung cancer solitary metastases:
systematic review and analysis of reported cases. Lung
Cancer 2012; 75: 9-14.
23) Barone M, Di Nuzzo D, Cipollone G, Camplese P, Mucilli F.
Oligometastatic non-small cell lung cancer (NSCLC): adrenal
metastases. Experience in a single institution. Updates Surg
2015; 67: 383-7.