2Department of Radiology, Istanbul Aydın University, Medical Faculty, Istanbul, Turkey
3Department of Pulmonology, Istanbul Aydın University, Medical Faculty, Istanbul, Turkey
4Department of Thoracic Surgery, Istanbul University, Medical Faculty, Istanbul, Turkey DOI : 10.26663/cts.2025.011
Summary
Background: Diaphragmatic elevation is among the earliest compensatory changes following lung resection; however, its extent and duration remain inadequately defined.Materials and Methods: A retrospective analysis was conducted on 198 patients who underwent surgical treatment for non-small cell lung cancer between January 2022 and June 2023. Of these, 102 patients who underwent upper or lower lobectomy were included in the study. Diaphragmatic position was assessed using chest radiographs obtained preoperatively and on postoperative days 1, 10, and 30. Measurements were performed by independent radiologists and compared according to surgical approach (VATS vs. thoracotomy), lobectomy location (upper vs. lower), and operative side (right vs. left).
Results: Significant diaphragmatic elevation was observed on the first postoperative day and persisted through day 10. The mean difference between preoperative and postoperative day 1 measurements was 29.16 ± 12.93 mm (p < 0.0001). Partial recovery was noted by day 10 (p < 0.001). Both hemidiaphragms showed similar patterns, although the right hemidiaphragm demonstrated greater recovery. No significant associations were identified between diaphragmatic elevation and surgical approach, lobectomy location, or laterality (p > 0.05 for all comparisons).
Conclusions: Diaphragmatic elevation represents a normal anatomical adaptation in the early postoperative period following lung resection. Defining its expected extent and duration may facilitate the early recognition of postoperative complications. Larger-scale studies are warranted to establish definitive reference criteria for postoperative diaphragmatic elevation.
Introduction
Lung cancer remains one of the most frequently diagnosed malignancies worldwide, with an estimated 2.48 million new cases annually. It is also the leading cause of cancer-related mortality, accounting for approximately 1.79 million deaths each year [1,2].For patients with early-stage non-small cell lung cancer (NSCLC), surgical resection is the primary treatment modality and the cornerstone of thoracic oncologic surgery. Lobectomy with ipsilateral mediastinal lymph node dissection is considered the gold standard surgical approach for NSCLC [3]. In addition to lobectomy, other procedures such as bilobectomy, sleeve lobectomy, and pneumonectomy may be performed; in addition, sublobar resections, including segmentectomy and wedge resection, are also indicated in selected cases [4-6]. In patients with locally advanced NSCLC, radical resection may be extended to involve adjacent structures such as the chest wall, major vessels, or the atrium [7].
Lung resections result in notable anatomical changes, including narrowing of the intercostal spaces, mediastinal shift toward the operated side, diaphragmatic elevation, and compensatory expansion of the contralateral lung [8]. These alterations are often visible on postoperative imaging and must be distinguished from pathological findings to avoid misdiagnosis and to promptly identify potential complications.
The aim of this study was to investigate the extent and duration of diaphragmatic elevation following lobectomy and to evaluate variations based on surgical side and type of resection. By defining normative postoperative patterns of diaphragmatic elevation, we seek to contribute to the early detection of postoperative complications.
Methods
This study was designed as a retrospective observational analysis. Between January 1, 2022, and June 30, 2023, a total of 198 patients who underwent surgical treatment for non-small cell lung cancer (NSCLC) at our institution were retrospectively reviewed. Patients who underwent segmentectomy (n = 16), wedge resection (n = 12), middle lobectomy (n = 7), bilobectomy (n = 11), or pneumonectomy (n = 8), as well as those with prolonged postoperative air leak (n = 9), those requiring reoperation (n = 6), or those who received neoadjuvant or adjuvant chemotherapy (n = 18) or chemo-immunotherapy (n = 9), were excluded. A total of 102 patients, including 63 female, met the inclusion criteria and were included in the final analysis. The study was conducted in accordance with the Declaration of Helsinki and ethical approval was obtained from the institutional review board.
Chest X-ray evaluation
The diaphragmatic position was evaluated using standard
upright posteroanterior chest radiographs obtained
during full inspiration at four time points: preoperatively
(within one week prior to surgery) and on postoperative
days 1, 10, and 30. Independent radiologists, blinded
to clinical information and surgical details, measured
the vertical distance (in millimeters) from the highest
point of the hemidiaphragm to the thoracic apex on the
ipsilateral side. Diaphragmatic elevation was compared
across subgroups according to surgical approach (VATS
vs. thoracotomy), operative side (right vs. left), and lobectomy
location (upper vs. lower).
All radiographs were acquired with patients standing in full inspiration and facing the cassette. The x-ray tube was positioned 6 feet behind the patient, and patients were instructed to hold onto handles beside the cassette to move their arms away from the chest. Images were obtained during deep inspiration [9].
Outcome measures
The primary outcome measure was the change in the
vertical distance from the diaphragm apex to the thoracic
apex across time points. As illustrated in Figure
1, measurements were taken from chest radiographs at
four time points: preoperative (A), postoperative day
1 (B), day 10 (C), and day 30 (D). Differences in diaphragm
position were calculated between the following
pairs: day 1 vs. preoperative, day 10 vs. preoperative,
day 30 vs. preoperative, day 10 vs. day 1, day 30 vs. day
1, and day 30 vs. day 10.
Statistical Analysis
Statistical analyses were conducted using Python
(version 3.12.4) with the pandas (2.2.3), NumPy
(1.26.4), SciPy (1.11.4), Matplotlib (3.10.1), and Seaborn
(0.13.2) libraries. For each timepoint comparison, normality and variance homogeneity were assessed before
selecting the appropriate statistical test. The Shapiro-
Wilk test was used to assess normality, and Levene’s
test was employed to evaluate homogeneity of variances.
For normally distributed data, paired t-test was used
for within-subject comparisons, and independent t-test
(or Welch’s modification for unequal variances) for between-
group comparisons. For non-normally distributed
data, the Wilcoxon signed-rank test (paired samples)
and the Mann-Whitney U test (unpaired samples) were
applied. One-sample comparisons against reference
values were done by t-test or Wilcoxon test, depending
on the distribution.
Subgroup analyses were performed according to surgical technique (VATS vs. thoracotomy), lobectomy location (upper vs. lower), and operative side (left vs. right). Statistical significance was set at p < 0.05. Significance levels in figures are denoted as follows: *p < 0.05, **p < 0.01, and ***p < 0.001.
Results were visualized using bar charts with standard deviation error bars. Comparative analyses were presented using side-by-side visualizations with direct statistical annotations. Where applicable, dual y-axes were used to display both absolute distances and percentages normalized to preoperative values. All statistical comparisons between time points and subgroups were annotated in the corresponding figures.
Results
Between January 1, 2022, and June 30, 2023, we retrospectively analyzed 102 consecutive patients who underwent pulmonary lobectomy for non-small cell lung cancer at our institution. As detailed in Table 1, the study population included 63 females (61.8%) and 39 males (38.2%). Video-assisted thoracoscopic surgery (VATS) was performed in 71 patients (69.6%), while conventional thoracotomy was used in 31 cases (30.4%). The right hemithorax was involved in 69 procedures (67.6%) and the left in 33 (32.4%). Upper lobectomy was the most common procedure (71 patients, 69.6%), followed by lower lobectomy (31 patients, 30.4%).Table 1: Baseline characteristics of the patients and the procedures.
Changes in diaphragm position
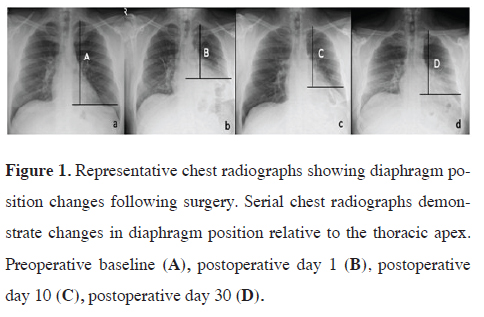
Figure 1 displays representative chest radiographs demonstrating
the positional changes of the diaphragm relative to the thoracic apex. Figure 1A shows the preoperative
baseline position. On postoperative day 1 (Figure
1B), marked diaphragmatic elevation is evident. Changes
observed on day 10 (Figure 1C) and on day 30 (Figure
1D) are also shown.
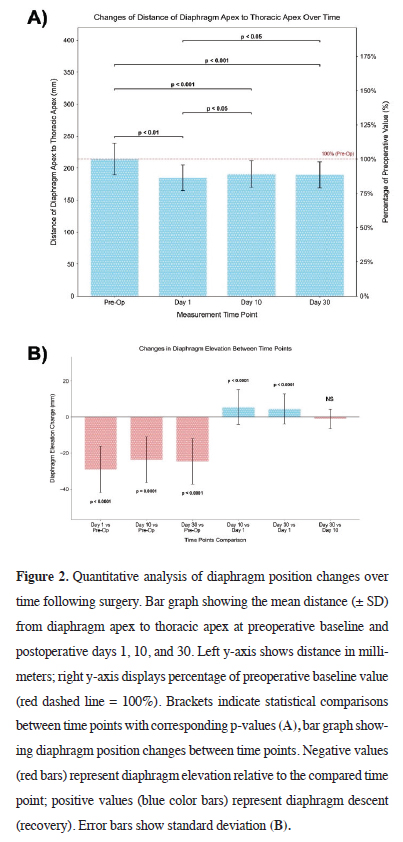
Figure 2A presents the mean vertical distance from the diaphragm apex to the thoracic apex at each time point. The baseline preoperative value (214.19 ± 24.92 mm) significantly decreased on postoperative day 1 (185.03 ± 20.01 mm; 86.39% of baseline; p < 0.01). On day 10, a modest increase was recorded (190.52 ± 20.71 mm; 88.95% of baseline), which remained significantly lower than preoperative values (p < 0.001) but higher than day 1 (p < 0.05). Day 30 measurements (189.53 ± 20.54 mm; 88.49% of baseline) were also significantly reduced compared to baseline (p < 0.001) and differed significantly from day 1 (p < 0.05).
 Click Here to Zoom |
Figure 1: Representative chest radiographs showing diaphragm position changes following surgery. Serial chest radiographs demonstrate changes in diaphragm position relative to the thoracic apex. Preoperative baseline (A), postoperative day 1 (B), postoperative day 10 (C), postoperative day 30 (D). |
 Click Here to Zoom |
Figure 2: Quantitative analysis of diaphragm position changes over time following surgery. Bar graph showing the mean distance (± SD) from diaphragm apex to thoracic apex at preoperative baseline and postoperative days 1, 10, and 30. Left y-axis shows distance in millimeters; right y-axis displays percentage of preoperative baseline value (red dashed line = 100%). Brackets indicate statistical comparisons between time points with corresponding p-values (A), bar graph showing diaphragm position changes between time points. Negative values (red bars) represent diaphragm elevation relative to the compared time point; positive values (blue color bars) represent diaphragm descent (recovery). Error bars show standard deviation (B). |
Figure 2B illustrates the magnitude of change across intervals. The most pronounced elevation occurred immediately after surgery, with a mean diaphragm elevation of 29.16 ± 12.93 mm from baseline to day 1 (p < 0.0001). A partial recovery of 5.49 ± 9.55 mm occurred between days 1 and 10 (p < 0.0001), and 4.50 ± 8.35 mm from day 1 to day 30 (p < 0.0001). No significant difference was detected between days 10 and 30 (-0.99 ± 5.37 mm; p > 0.05).
Left versus right hemidiaphragm
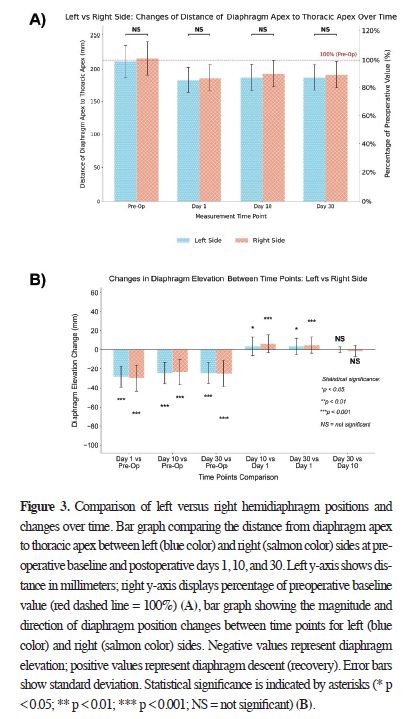
Figure 3A shows the quantitative analysis of diaphragm
apex to thoracic apex distance on the left (n = 33) and right
(n = 69) sides across all time points. Preoperatively, the
right hemidiaphragm exhibited higher distance (215.67 ±
25.13 mm) than the left (211.09 ± 24.57 mm), but the difference
was not statistically significant (p > 0.05).
 Click Here to Zoom |
Figure 3: Comparison of left versus right hemidiaphragm positions and changes over time. Bar graph comparing the distance from diaphragm apex to thoracic apex between left (blue color) and right (salmon color) sides at preoperative baseline and postoperative days 1, 10, and 30. Left y-axis shows distance in millimeters; right y-axis displays percentage of preoperative baseline value (red dashed line = 100%) (A), bar graph showing the magnitude and direction of diaphragm position changes between time points for left (blue color) and right (salmon color) sides. Negative values represent diaphragm elevation; positive values represent diaphragm descent (recovery). Error bars show standard deviation. Statistical significance is indicated by asterisks (* p < 0.05; ** p < 0.01; *** p < 0.001; NS = not significant) (B). |
On postoperative day 1, both sides exhibited significant elevation- 183.12 ± 19.38 mm (left, 86.75% of baseline) and 185.94 ± 20.38 mm (right, 86.22% of baseline)-with no significant side-to-side difference (p > 0.05). By day 10, the right hemidiaphragm exhibited higher distance, but without a statistically significant difference (192.35 ± 20.82 mm vs. 186.70 ± 20.24 mm; p > 0.05). Similar findings were observed on day 30: 190.87 ± 20.85 mm (right) and 186.73 ± 19.89 mm (left; p > 0.05). Overall, diaphragm elevation patterns were bilaterally proportional with no statistically significant differences at any time point (p > 0.05).
As shown in Figure 3B, both sides experienced significant diaphragmatic elevation compared to preoperative values. The most substantial changes were seen on day 1: -27.97 ± 11.06 mm (left) and -29.72 ± 13.77 mm (right; p < 0.001 for both). This persisted on day 10 (left: -24.39 ± 11.33 mm; right: -23.32 ± 13.35 mm; p < 0.001) and day 30 (left: -24.36 ± 10.98 mm; right: -24.80 ± 13.49 mm; p < 0.001).
In Figure 3B, analysis of recovery patterns between consecutive time points revealed significant improvement in diaphragm position from day 1 to day 10 on both sides: 3.58 ± 9.60 mm for the left (p < 0.05) and 6.41 ± 9.45 mm for the right (p < 0.001), with the right side exhibiting more pronounced recovery. No significant changes were observed between days 10 and 30 for either the left (0.03 ± 2.82 mm, p > 0.05) or right side (-1.48 ± 6.19 mm, p > 0.05). Change in elevation between day 1 and day 30 was significant for both the left (3.61 ± 8.49 mm, p < 0.05) and right sides (4.93 ± 8.31 mm, p < 0.001). Although both sides showed improvement during this period, the diaphragm position remained significantly elevated compared to preoperative measurements on day 30. Notably, the right side exhibited a more statistically significant degree of recovery between day 1 and day 30 (p < 0.001) compared to the left side (p < 0.05).
Comparison by surgical approach
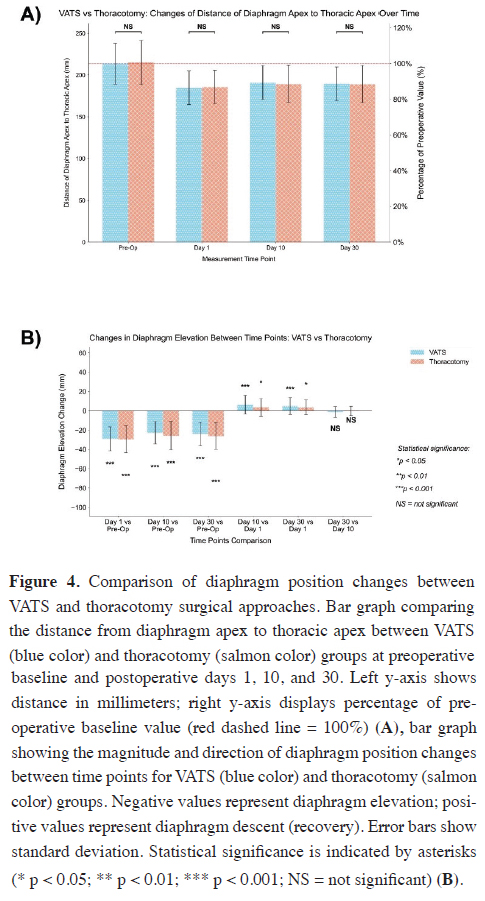
Figure 4A compares diaphragm position by surgical approach.
Baseline values for diaphragm-to-thoracic apex distance
were comparable between the VATS (213.73 ± 24.38
mm) and thoracotomy groups (215.23 ± 26.49 mm; p > 0.05).
In Figure 4A, both groups demonstrated a similar pattern of diaphragm elevation following surgery. On day 1, elevation occurred in both groups (VATS: 184.73 ± 20.13 mm, 13.6% reduction; thoracotomy: 185.71 ± 20.03 mm, 13.7% reduction), with no significant difference (p > 0.05) (Figure 4A).
 Click Here to Zoom |
Figure 4: Comparison of diaphragm position changes between VATS and thoracotomy surgical approaches. Bar graph comparing the distance from diaphragm apex to thoracic apex between VATS (blue color) and thoracotomy (salmon color) groups at preoperative baseline and postoperative days 1, 10, and 30. Left y-axis shows distance in millimeters; right y-axis displays percentage of preoperative baseline value (red dashed line = 100%) (A), bar graph showing the magnitude and direction of diaphragm position changes between time points for VATS (blue color) and thoracotomy (salmon color) groups. Negative values represent diaphragm elevation; positive values represent diaphragm descent (recovery). Error bars show standard deviation. Statistical significance is indicated by asterisks (* p < 0.05; ** p < 0.01; *** p < 0.001; NS = not significant) (B). |
At postoperative day 10, partial recovery of diaphragm position was observed in both groups, with mean distances of 191.01 ± 20.05 mm in the VATS group and 189.39 ± 22.44 mm in the thoracotomy group, corresponding to 89.4% and 88.0% of preoperative values, respectively. No significant difference was found between the two surgical approaches (p > 0.05).
By postoperative day 30, the diaphragm position measured 189.72 ± 20.00 mm in the VATS group and 189.10 ± 22.06 mm in the thoracotomy group, representing 88.8% and 87.9% of baseline values, respectively. Again, no significant difference was observed between the groups (p > 0.05). These findings suggest that the choice of surgical approach (VATS or thoracotomy) did not significantly affect the extent of postoperative diaphragm elevation or its recovery pattern over the 30-day follow-up period.
Figure 4B illustrates diaphragmatic changes within and between time points by surgical technique. Both groups showed significant decreases in distances from baseline on all postoperative days (VATS: -29.00 ± 12.49 mm, -22.72 ± 11.74 mm, -24.01 ± 12.25 mm; thoracotomy: -29.52 ± 14.08 mm, -25.84 ± 14.62 mm, -26.13 ± 13.72 mm; all p < 0.001).
In Figure 4B, both groups exhibited significant partial recovery of diaphragmatic elevation between postoperative day 1 and subsequent time points. In the VATS group, the diaphragm position improved by 6.28 ± 9.63 mm between days 1 and 10 (p < 0.001) and by 4.99 ± 8.58 mm between days 1 and 30 (p < 0.001). Similarly, the thoracotomy group demonstrated significant recovery, with increases of 3.68 ± 9.24 mm from day 1 to day 10 (p < 0.05) and 3.39 ± 7.80 mm from day 1 to day 30 (p < 0.05). No significant change occurred between days 10 and 30 in either group (VATS: -1.30 ± 5.58 mm; thoracotomy: -0.29 ± 4.88 mm; p > 0.05) (Figure 4B).
Upper vs. lower lobectomy
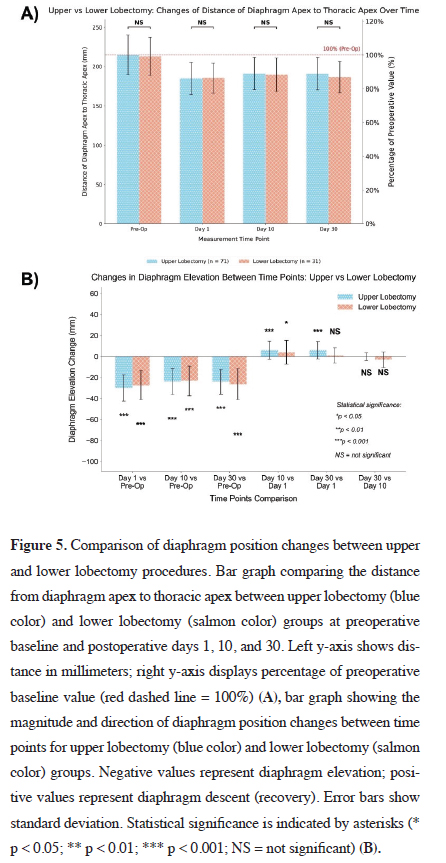
In Figure 5A, the distance from the diaphragm apex to
the thoracic apex was compared between patients who
underwent upper lobectomy (n = 71) and those who underwent
lower lobectomy (n = 31), at four time points.
At baseline, both groups exhibited similar preoperative
diaphragm positions (upper: 214.79 ± 25.34 mm; lower:
212.81 ± 24.27 mm; p > 0.05).
On postoperative day 1, both groups demonstrated significant decreases in diaphragm-to-thoracic apex distance, indicating diaphragmatic elevation. The mean distances were 184.83 ± 20.56 mm for the upper lobectomy group and 185.48 ± 19.00 mm for the lower lobectomy group, with no statistically significant difference between them (p > 0.05). This pattern persisted on day 10 (upper: 190.90 ± 20.69 mm; lower: 189.65 ± 21.07 mm; p > 0.05) and day 30 (upper: 190.80 ± 20.80 mm; lower: 186.61 ± 19.95 mm; p > 0.05). Notably, by day 30, neither group had returned to their baseline diaphragm position, with the diaphragm-to-apex distance remaining at approximately 89% of the preoperative value in the upper lobectomy group and 88% in the lower lobectomy group.
Figure 5B presents the changes in diaphragm elevation across time points for upper and lower lobectomy patients. Both groups showed highly significant diaphragmatic elevation immediately after surgery, with diaphragm-tothoracic apex distance decreases of -29.96 ± 12.58 mm in the upper lobectomy group and -27.32 ± 13.73 mm in the lower lobectomy group (p < 0.001, for both). This elevation persisted through postoperative days 10 and 30, with measurements remaining significantly different from baseline in both groups (all p < 0.001).
Between days 1 and 10, both groups exhibited statistically significant recovery: 6.07 ± 8.73 mm for the upper lobectomy group (p < 0.001) and 4.16 ± 11.24 mm for the lower lobectomy group (p < 0.05) (Figure 5B). However, a notable difference emerged between days 1 and 30. The upper lobectomy group showed continued significant improvement (5.97 ± 8.47 mm, p < 0.001), whereas the lower lobectomy group demonstrated a smaller, statistically non-significant change (1.13 ± 7.10 mm, p > 0.05). Between days 10 and 30, neither group showed significant recovery (upper: -0.10 ± 3.77 mm, p > 0.05; lower: -3.03 ± 7.60 mm, p > 0.05).
 Click Here to Zoom |
Figure 5: Comparison of diaphragm position changes between upper and lower lobectomy procedures. Bar graph comparing the distance from diaphragm apex to thoracic apex between upper lobectomy (blue color) and lower lobectomy (salmon color) groups at preoperative baseline and postoperative days 1, 10, and 30. Left y-axis shows distance in millimeters; right y-axis displays percentage of preoperative baseline value (red dashed line = 100%) (A), bar graph showing the magnitude and direction of diaphragm position changes between time points for upper lobectomy (blue color) and lower lobectomy (salmon color) groups. Negative values represent diaphragm elevation; positive values represent diaphragm descent (recovery). Error bars show standard deviation. Statistical significance is indicated by asterisks (* p < 0.05; ** p < 0.01; *** p < 0.001; NS = not significant) (B). |
Discussion
Lung cancer remains the leading cause of cancer-related mortality worldwide. According to GLOBOCAN 2022, there were approximately 2.48 million new cases and 1.81 million deaths attributed to lung cancer, reflecting a nearly 20% increase in incidence compared to previous data [1,10]. Surgical resection remains the cornerstone of treatment for stage I and II non-small cell lung cancer (NSCLC) [11,12]. Consequently, the growing incidence of lung cancer and the increased application of surgical management have led to a greater number of patients undergoing lung resection.Postoperative follow-up in these patients who have undergone lung resection typically begins with chest radiographs, while computed tomography (CT) is more commonly used for evaluating recurrence and late complications. Radiological findings in the early postoperative period often reflect expected anatomical adaptations to lung resection, such as minor pneumothorax or hydropneumothorax, parietal emphysema, hemithorax retraction. Over few days to weeks, additional compensatory changes include expansion of the remaining lung lobes, blunting of the costodiaphragmatic angle, and elevation of the diaphragm and hilar structures, as well as a mediastinal shift towards the operated side [5]. These changes result in progressive anatomical modifications of the thoracic cavity depending on the type of resection. Correct interpretation of these normal postoperative radiological findings is essential to avoid misdiagnosis and to detect early complications [13]. For instance, excessive diaphragmatic elevation may suggest phrenic nerve injury, whereas insufficient elevation may point to complications such as pneumothorax, hemothorax, or expansion defects.
Following lung resection, the diaphragm rapidly adapts to the changing thoracic volume, resulting in early elevation. However, this phenomenon is largely assessed subjectively, and there is no widely accepted quantitative standard for its evaluation [14]. Furthermore, the expected duration and extent of diaphragmatic elevation have not been clearly defined in the literature. In our study, we observed that most of the compensatory diaphragmatic elevation occurred within the first 10 postoperative days (Figures 2A, B).
The diaphragm is a striated muscle that separates the thoracic and abdominal cavities, attaching from the xiphoid process of the sternum to the 7th through 12th ribs, with a central tendon shaped like a dome. Its primary function is assisting ventilation [9,15,16]. On a posteroanterior chest X-ray, the dome of the diaphragm is positioned at the level of the 5th to 7th ribs along the midclavicular line. In most individuals, the right hemidiaphragm lies 1.5 to 2.5 cm higher than the left hemidiaphragm [9,17]. Diaphragmatic eventration is defined as abnormal elevation of a portion or the entire hemidiaphragm due to loss of muscle or nerve function, while anatomical continuity is preserved [18]. This condition is typically partial, involving one-third to one-half of the hemidiaphragm, and appears radiographically as a smoothly contoured elevation. Eventration is more frequently observed on the left side but may progress to total diaphragmatic elevation over time [9]. Diaphragmatic elevation observed after thoracic surgery is caused by the retraction of the hemithorax, whereas diaphragmatic elevation developing after cardiac surgery is due to phrenic nerve damage and the mechanism of occurrence is completely different [19]. In our study, compensatory elevation was significantly greater on the right side (Figure 3B).
Although lung resection has traditionally been performed via thoracotomy, advances in camera systems and surgical instruments have improved the feasibility of video-assisted thoracic surgery (VATS), allowing for smaller incisions, shorter operative times, and enhanced safety. Consequently, the use of VATS has become increasingly common [10]. Thoracoscopic surgery offers several advantages, including shorter hospital stays, reduced chest tube duration, lower postoperative pain, better preservation of pulmonary function, decreased cytokine release, and a lower overall complication rate [11]. In our study, no significant effect of surgical technique on diaphragmatic elevation was observed (Figure 4B). The diaphragm movement that develops due to thoracic surgery is a compensatory elevation, it is not affected by the inflammatory response created by the surgical technique.
In conclusion, diaphragmatic elevation in patients undergoing lung resection occurs predominantly on the first postoperative day and continues until approximately day 10. The absence or excessive presence of this elevation may serve as a supportive indicator for the early detection of postoperative complications. Large-scale studies are needed to determine the standardization of postoperative diaphragm elevation.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect
to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Ethics approval
This study was approved by Istanbul Aydin University
Non-invasive Research Ethics Committee with protocol
number 102-2025.
Authors’ contribution
IK,BK; made substantial contributions to the design
of the work, IK,BK,FST; made the analysis of data,
FST,BO; made the creation of new software used in the
work, IK,BK,FST; have drafted the work, IK,BO; revised
it. All authors read and approved the fnal manuscript.
Reference
1) Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram
I et al. Global cancer statistics 2022: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers
in 185 countries. CA Cancer J Clin 2024; 74: 229-63.
2) Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung
cancer. Lancet 2021; 398: 535-54.
3) Aokage K, Suzuki K, Saji H, Wakabayashi M, Kataoka T,
Sekino Y et al. Segmentectomy for ground-glass-dominant
lung cancer with a tumour diameter of 3 cm or less including
ground-glass opacity (JCOG1211): a multicentre, single-arm,
confirmatory, phase 3 trial. Lancet Respir Med 2023; 11: 540-9.
4) Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K
et al. Segmentectomy versus lobectomy in small-sized peripheral
non-small-cell lung cancer (JCOG0802/WJOG4607L): a
multicentre, open-label, phase 3, randomised, controlled, noninferiority
trial. Lancet 2022; 399: 1607-17.
5) Burel J, El Ayoubi M, Baste JM, Garnier M, Montagne F,
Dacher JN, et al. Surgery for lung cancer: postoperative changes
and complications-what the Radiologist needs to know. Insights
Imaging 2021; 12: 116.
6) Berthet JP, Paradela M, Jimenez MJ, Molins L, Gomez-Caro
A. Extended sleeve lobectomy: one more step toward avoiding
pneumonectomy in centrally located lung cancer. Ann Thorac
Surg 2013; 96: 1988-97.
7) Speicher PJ, Englum BR, Ganapathi AM, Onaitis MW,
D'Amico TA, Berry MF. Outcomes after treatment of 17,378
patients with locally advanced (T3N0-2) non-small-cell lung
cancer. Eur J Cardiothorac Surg 2015; 47: 636-41.
8) Simsek B, Ozyuksel A, Saygi M, Bilal MS. Plication for diaphragm
paralysis after paediatric cardiac surgery: a single-centre
experience. Cardiol Young 2023; 33: 2087-93.
9) Kalra MK. Thoracic imaging. In: Shepard JAO, Abbott GF,
Ackman JB, et al., eds. Thoracic Imaging: The Requisites.
Philadelphia: Elsevier; 2019.
10) Kolbas I, Tezel C, Ozturk A, Alpay L, Evman S. Does VATS
Lobectomy Avoid Delay and Increase Compliance in Adjuvant
Chemotherapy? Turk J Oncol 2022; 37: 388-93.
11) Howington JA, Blum MG, Chang AC, Balekian AA, Murthy
SC. Treatment of stage I and II non-small cell lung cancer:
Diagnosis and management of lung cancer, 3rd ed: American
College of Chest Physicians evidence-based clinical practice
guidelines. Chest 2013; 143(5 Suppl): e278S-e313S.
12) Paul S, Altorki NK, Sheng S, Lee PC, Harpole DH, Onaitis MW
et al. Thoracoscopic lobectomy is associated with lower morbidity
than open lobectomy: a propensity-matched analysis from the
STS database. J Thorac Cardiovasc Surg 2010; 139: 366-78.
13) Bommart S, Berthet JP, Durand G, Ghaye B, Pujol JL, Marty-
Ane C et al. Normal postoperative appearances of lung cancer.
Diagn Interv Imaging 2016; 97: 1025-35.
14) Ventura L, Zhao W, Chen T, Wang Z, Feng J, Gu Z, et al. Significant
diaphragm elevation suggestive of phrenic nerve injury
after thoracoscopic lobectomy for lung cancer: an underestimated
problem. Transl Lung Cancer Res 2020; 9: 1822-31.
15) Hamer OW, Sirlin CB, Strotzer M, Brisch I, Zorger N, Feuerbach
S et al. Chest radiography with a flat-panel detector: image
quality with dose reduction after copper filtration. Radiology
2005; 237: 691-700.
16) Nason LK, Walker CM, McNeeley MF, Burivong W, Fligner
CL, Godwin JD. Imaging of the diaphragm: anatomy and function.
Radiographics 2012; 32: E51-70.
17) Shah-Mirany J, Schmitz GL, Watson RR. Eventration of the
diaphragm. Physiologic and surgical significance. Arch Surg
1968; 96: 844-50.