2Faculty of Medicine, Pharmacy, and Dental Medicine, Sidi Mohamed Ben Abdellah University, Fès, Morocco
3Department of Radiology, Hassan II University Hospital, Fès, Morocco DOI : 10.26663/cts.2025.015
Summary
Nodular ganglioneuroblastoma is an exceptionally rare neuroblastic neoplasm in the adult population. This report details a highly unusual case of posterior mediastinal ganglioneuroblastoma in a 55-year-old male who presented solely with posterior chest pain. Radiological investigations, including computed tomography and magnetic resonance imaging, demonstrated an encapsulated, highly cellular tumor in the left costovertebral groove at the D7 level, with no evidence of distant metastasis on positron emission tomography. The patient underwent successful surgical resection following preoperative embolization and experienced an uneventful postoperative recovery. Histopathological analysis confirmed the diagnosis of nodular ganglioneuroblastoma. In summary, ganglioneuroblastoma should be considered in the differential diagnosis of posterior mediastinal tumors, even in adult patients.Introduction
Nodular ganglioneuroblastoma (GNB) is a neuroblastic tumor of the sympathetic nervous system that derives from primordial neural crest cells [1]. It is the most common solid tumor in childhood. In the adult population, it is extremely rare, with less than 50 cases have been reported in the English literature [2]. According to the International Neuroblastoma Pathology Classification (INPC), it is defined as a stroma-rich or stromadominant tumor (such as ganglioneuroma or intermixed GNB) that contains one or more macroscopic nodules of stroma-poor neuroblastoma [3-5]. We present the clinical course and a review of adult-onset posterior mediastinal nodular ganglioneuroblastoma.Case Presentation
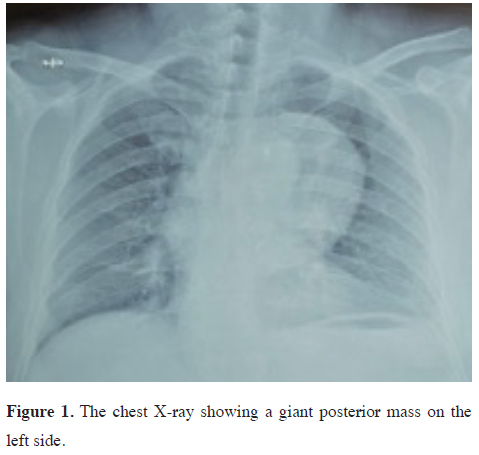
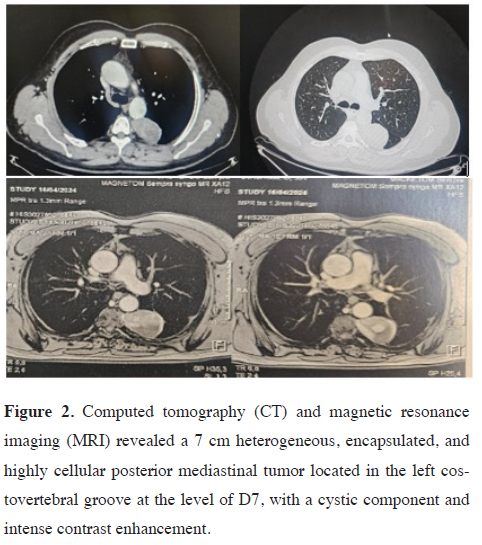
A 55-year-old man presented with a chief complaint of posterior chest pain radiating to the sternum. He had a recent history of hypertension and a history of tobacco use, having quit smoking 30 years ago after smoking for 2 years. He was otherwise in good health with no other significant symptoms. His surgical history was unremarkable, and there was no family history of tumors or neuroblastoma.Chest radiography and computed tomography (CT) revealed a 7 cm heterogeneous posterior mediastinal tumor with contrast enhancement (Figures 1,2). Magnetic resonance imaging (MRI) further demonstrated an encapsulated, highly cellular tumor located in the left costovertebral groove at the level of D7, with a cystic component and intense contrast enhancement. There was no evidence of extension or invasion of the aorta.
 Click Here to Zoom |
Figure 1: The chest X-ray showing a giant posterior mass on the left side. |
 Click Here to Zoom |
Figure 2: Computed tomography (CT) and magnetic resonance imaging (MRI) revealed a 7 cm heterogeneous, encapsulated, and highly cellular posterior mediastinal tumor located in the left costovertebral groove at the level of D7, with a cystic component and intense contrast enhancement. |
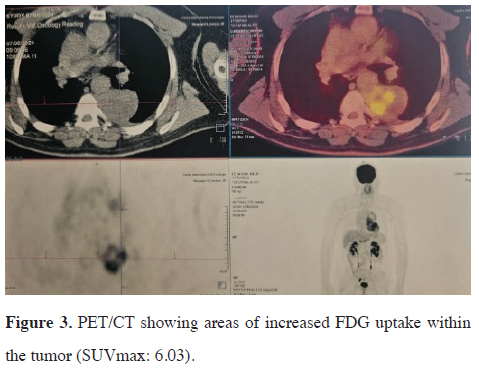
After a multidisciplinary discussion, the decision was made to perform laboratory analysis of catecholamine metabolites, PET scan and a preoperative embolization. Laboratory findings showed urine normetadrenaline at 269 nmol/24 h (normal range < 281 nmol/24 h), urine metadrenaline at 59 nmol/24 h (normal range < 159 nmol/24 h), and urine 3-ortho-methyldopa at 1981 nmol/24 h (normal range < 329 nmol/24 h). Positron emission tomography with fluorodeoxyglucose-computed tomography (FDG-PET/CT) revealed positive FDG uptake in the mediastinal mass lesion with areas corresponding to those enhanced on contrast CT (SUVmax: 6.03) (Figure 3). No abnormal FDG uptake was observed in other organs.
 Click Here to Zoom |
Figure 3: PET/CT showing areas of increased FDG uptake within the tumor (SUVmax: 6.03). |
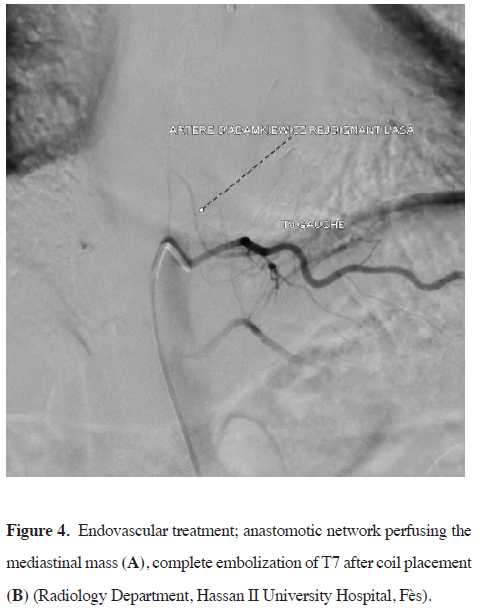
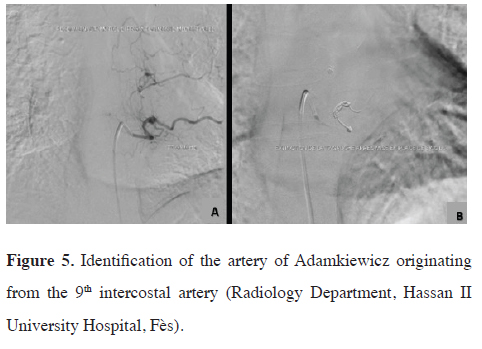
The patient underwent angiography and preoperative embolization of the 4th, 6th, and 7th intercostal arteries using coil placement to reduce blood inflow and minimize the risk of recanalization, with identification of the artery of Adamkiewicz originating from the 9th intercostal artery (Figures 4,5). The surgery was done 24 hours later.
 Click Here to Zoom |
Figure 4: Endovascular treatment; anastomotic network perfusing the mediastinal mass (A), complete embolization of T7 after coil placement (B) (Radiology Department, Hassan II University Hospital, Fès). |
 Click Here to Zoom |
Figure 5: Identification of the artery of Adamkiewicz originating from the 9th intercostal artery (Radiology Department, Hassan II University Hospital, Fès). |
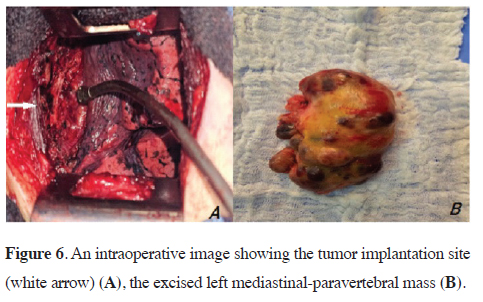
The tumor was removed via a conservative thoracotomy approach. The mass was encapsulated and relatively easy to excise, except near the D7 paraspinal region, where dissection was more challenging (Figure 6). There were no perioperative complications, including blood loss or neurological complications. We discharged the patient on the third postoperative day.
 Click Here to Zoom |
Figure 6: An intraoperative image showing the tumor implantation site (white arrow) (A), the excised left mediastinal-paravertebral mass (B). |
The pathological findings of the resected mass contained grossly visible neuroblastomatous nodules including ganglioneuromatous components. Immunohistochemical staining revealed tumor cells positive for chromogranin A, and synaptophysin. Ki-67 labeling index was 30% in the area. The patient underwent a thoraco-abdomino-pelvic CT scan three months after surgery, which returned normal. He is currently under close clinical follow-up (24 months) during which no recurrence or complications have been observed. Written informed consent was obtained from the patient for the use of the medical data in academic studies.
Discussion
Neuroblastic tumors generally occur in very young children, with a median age at diagnosis of 17 months. In adults, these tumors are extremely rare, with an incidence of less than 0.3 cases per million people annually [6-8]. They arise from tissues of the sympathetic nervous system, typically in the adrenal medulla or paraspinal ganglia, and can present as mass lesions in the neck, chest, abdomen, or pelvis [5]. According to the International Neuroblastoma Pathology Classification four types of NTs are distinguished: neuroblastoma, ganglioneuroblastoma- nodular, ganglioneuroblastoma-intermixed, and ganglioneuroma [4-8]. Based on the International Neuroblastoma Risk Group (INRG) analysis, the nodular subtype is associated with a poorer prognosis compared to other neuroblastic pathologies, such as neuroblastoma or the intermixed subtype of GNB [9]. Angelini et al identified two subgroups of patients with a very favorable prognosis: children younger than 547 days and those with non-stage 4 disease. In contrast, patients with stage 4 (distant metastases) nodular ganglioneuroblastoma demonstrated significantly worse outcomes (5-years EFS of 23%, and OS 35%) [10].The clinical presentation of neuroblastic tumors is highly variable, ranging from an asymptomatic mass to a primary tumor causing severe illness due to local invasion, widespread metastatic disease, or both. CT and MRI are the most commonly used imaging modalities for evaluating ganglioneuroblastoma. On CT, the appearance of these tumors ranges from well-marginated, oblong paravertebral masses with homogeneous enhancement to irregular, cystic, hemorrhagic, or locally invasive lesions [11,12]. On MRI, ganglioneuroblastoma appears hypointense or isointense on T1-weighted images, and hyperintense with a heterogeneous appearance on T2-weighted images [13]. PET/CT is an additional tool that can aid in diagnosing distant metastasis [12]. Elevated levels of serum neuron-specific enolase (NSE) and urinary catecholamine metabolites are observed in 90% of pediatric neuroblastoma (NB) cases. However, their elevation is rarely observed in adolescents, making them less reliable in this age group [14], which is the case for our patient.
In our experience, preoperative embolization is often recommended to optimize both preoperative and intraoperative conditions during extensive resective surgery. This technique minimizes blood flow to hypervascular tumors by targeting feeder vessels, which facilitates tumor shrinkage and alters its consistency. However, surgery should be performed within 24 hours of embolization. Identifying radiculomedullary arteries (RMAs), such as the artery of Adamkiewicz, is critical to prevent inadvertent embolization of spinal arteries and avoid severe complications [15,16].
Preoperative embolization of large mediastinal tumors with complex blood supplies is a well-established approach. Puma et al. reported three cases of highly vascularized giant thoracic sarcomas treated with preoperative embolization. In all cases, tumor size was reduced, and the resulting perilesional edema facilitated surgical dissection [17,18].
Biopsy can be useful for establishing a preoperative diagnosis [19] however, false-negative results may occur. Ganglioneuroblastomas can look like a neuroblastoma in a partial ganglioneuroma stroma [20].
Early and radical surgery is the best treatment available for mediastinal GNBs [21,22]. In adults, prognosis depends on surgical margin resection. According to Koike et al, adult patients with ganglioneuroblastomas that were only partially resected (R1 or R2) or left unresected had a survival period of less than 24 months [23]. In addition, tumor size at diagnosis may be associated with prognosis in adult GNB. Tumors larger than 8 cm in diameter tend to metastasize to distant organs [24].
There is no established evidence regarding the effectiveness of chemotherapy or radiotherapy in the survival of patients with GNB or neuroblastic tumor. This may be related to the fact that adults have a poorer tolerance to chemotherapy compared to that of children [25]. Further studies are required to confirm these observations.
In conclusion, patients with nodular GNB tumors represent a small subgroup characterized by significant heterogeneity in outcomes, similar to the overall neuroblastoma (NB) population. GNB should be considered a potential posterior mediastinal tumor, even in adult patients. The curative treatment is complete surgical resection; however, careful follow-up is necessary to monitor for metastasis and recurrence.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect
to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Author contribution
IA,HH,ML; conceptualization, IA,MT,KEA; data collection
and patient management, MM; radiological
analysis, IA; writing – original draft, HH,ML; co-writing
– review & editing:, MS,YO; supervision. All authors
contributed significantly to the drafting and revision
of the manuscript and approved the final version.
Acknowledgements
The authors wish to thank the Departments of Radiology
and Pathology at Hassan II University Hospital in
Fès for their support and collaboration in the management
of this case.
Reference
1) Kilton LJ, Aschenbrener C, Burns CP. Ganglioneuroblastoma
in adults. Cancer 1976; 37: 974-83.
2) Sekiguchi N, Noguchi T, Fukushima T, Kobayashi T, Ozawa T, Sato
Y et al. Posterior mediastinal ganglioneuroblastoma in an adolescent:
A case report and review. Thorac Cancer 2020; 11: 451-5.
3) Peuchmaur M, d'Amore ES, Joshi VV, Hata J, Roald B, Dehner
LP et al. Revision of the International Neuroblastoma Pathology
Classification: confirmation of favorable and unfavorable
prognostic subsets in ganglioneuroblastoma, nodular. Cancer
2003; 98: 2274-81.
4) Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B,
Stram DO, Gerbing RB, Lukens JN, Matthay KK, Castleberry
RP. The International Neuroblastoma Pathology Classification
(the Shimada system). Cancer 1999; 86: 364-72.
5) Shimada H, DeLellis RA, Marx A. Neuroblastic tumours of the
adrenal gland. In: Lloyd RV, Osamura RY, Kloppel G, Rosai J,
editors. WHO Classification of Tumours of Endocrine Organs.
4th ed. Lyon: IARC Press; 2017:196-203.
6) Choi JH, Ro JY. Mediastinal neuroblastoma, ganglioneuroblastoma,
and ganglioneuroma: Pathology review and diagnostic
approach. Semin Diagn Pathol 2022; 39: 120-30.
7) Esiashvili N, Goodman M, Ward K, Marcus RB Jr, Johnstone
PA. Neuroblastoma in adults: Incidence and survival analysis
based on SEER data. Pediatr Blood Cancer 2007; 49: 41-6.
8) Lam AK. Update on Adrenal Tumours in 2017 World Health
Organization (WHO) of Endocrine Tumours. Endocr Pathol
2017; 28: 213-27.
9) Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G,
Holmes K, et ; INRG Task Force. The International Neuroblastoma
Risk Group (INRG) staging system: an INRG Task Force
report. J Clin Oncol 2009; 27: 298-303.
10) Angelini P, London WB, Cohn SL, Pearson AD, Matthay KK,
Monclair T et. Characteristics and outcome of patients with
ganglioneuroblastoma, nodular subtype: a report from the
INRG project. Eur J Cancer 2012; 48: 1185-91.
11) Lee JY, Lee KS, Han J, Yoon HK, Kim TS, Han BK et al. Spectrum
of neurogenic tumors in the thorax: CT and pathologic
findings. J Comput Assist Tomogr 1999; 23: 399-406.
12) Mousa AM, Shokouh-Amiri MH, Shah LM, Garzon S, Xie
KL. Adult-onset ganglioneuroblastoma of the posterior mediastinum
with osseous metastasis. Radiol Case Rep 2020; 15:
1676-82.
13) Jain N, Halbert J, Patel PA, Biassoni L, Anderson J, Sebire N
et al. Importance of Magnetic Resonance Imaging With Diffusion-
weighted Imaging in Guiding Biopsy of Nodular Ganglioneuroblastoma:
A Case Report. J Pediatr Hematol Oncol 2021;
43: e130-e135.
14) Franks LM, Bollen A, Seeger RC, Stram DO, Matthay KK.
Neuroblastoma in adults and adolescents: an indolent course
with poor survival. Cancer 1997; 79: 2028-35.
15) Ashour R, Aziz-Sultan A. Preoperative tumor embolization.
Neurosurg Clin N Am 2014; 25: 607-7.
16) Chatani S, Haimoto S, Sato Y, Hasegawa T, Murata S, Yamaura
H, Inaba Y. Preoperative embolization of spinal metastatic
tumor: The use of selective computed tomography angiography
for the detection of radiculomedullary arteries. Spine Surg
Relat Res 2021; 5: 284-291.
17) Liu FY, Wang MQ, Duan F, Wang ZJ. Combined embolization
and surgical resection of a giant mediastinal tumor. Thorac Cardiovasc
Surg 2014; 62: 265–269.
18) Puma F, Cardini CL, Passalacqua G, Ragusa M. Preoperative
embolization in surgical management of giant thoracic sarcomas.
Eur J Cardiothorac Surg 2008; 33: 127-9.
19) Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V,
Castelberry RP et al. Revisions of the international criteria for
neuroblastoma diagnosis, staging, and response to treatment. J
Clin Oncol 1993; 11: 1466-77.
20) Alexander N, Sullivan K, Shaikh F, Irwin MS. Characteristics
and management of ganglioneuroma and ganglioneuroblastoma-
intermixed in children and adolescents. Pediatr Blood Cancer
2018; 65: e26964.
21) Decarolis B, Simon T, Krug B, Leuschner I, Vokuhl C, Kaatsch
et al. Treatment and outcome of Ganglioneuroma and Ganglioneuroblastoma
intermixed. BMC Cancer 2016; 16: 542.
22) Fatimi SH, Bawany SA, Ashfaq A. Ganglioneuroblastoma of
the posterior mediastinum: A case report. J Med Case Rep.
2011; 5: 322.
23) Koike K, Iihara M, Kanbe M, Omi Y, Aiba M, Obara T. Adulttype
ganglioneuroblastoma in the adrenal gland treated by a laparoscopic
resection: report of a case. Surg Today 2003; 33: 785-90.