2Department of Chest Disease, Süreyyapaşa Chest Disease and Thoracic Surgery, Education and Research Hospital, İstanbul, Turkey DOI : 10.26663/cts.2017.0017
Summary
Currently, thoracentesis is used to drain accumulated fluids in chest cavity such as; pleural effusion, hemothorax and chylothorax and also for diagnosis and treatment of diseases. It is a simple and reliable treatment method which can be used for air drainage in partial pneumothorax cases. After thoracentesis, some complications can occur such as pneumothorax, hemothorax, reexpansion pulmonary edema and organ laceration. Among these complications, pneumothorax is the most frequently seen. In this case report we presented a patient with lung cancer where bilateral pneumothorax developed after thoracentesis.Introduction
The pneumothorax, known since from ancient times, was first described by Boerhaave in 1724. However, the practice of routine tube thoracostomy began in 1950s. Pneumothorax is defined as the accumulation of free air between the parietal and visceral pleural foliage [1].The most common complication after thoracentesis is pneumothorax. Hemothorax, pleural infection, organ injuries, vasovagal reaction and soft tissue infection may also be considered as other complications [2]. Also, after therapeutic thoracentesis, additional reexpansion pulmonary edema and hypovolemia may occur [3]. Because of the low risk of pneumothorax after thoracentesis, it is not possible to come to a definite conclusion regarding the clinical benefits of the routine chest X-ray scans following thoracentesis [4].
In this case report we presented a patient with squamous cell lung carcinoma where bilateral pneumothorax developed after thoracentesis.
Case Presentation
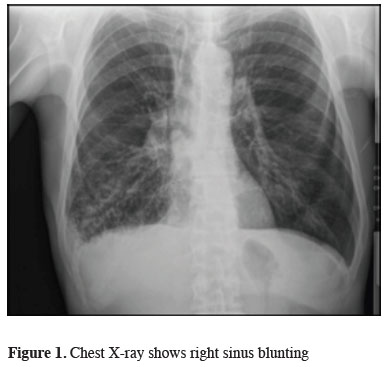
A 64-years-old male patient was admitted to our clinic with a complaint of shortness of breath. Posterioanterior (PA) chest X-ray shows right sinus blunting and increased density consistent with right central colon (Figure 1).
 Click Here to Zoom |
Figure 1: Chest X-ray shows right sinus blunting |
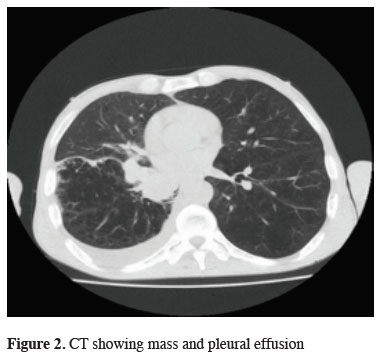
On computed tomography of the patient, 43 x 41 mm mass and pleural effusion are present in the right lower lobe of the lung (Figure 2).
 Click Here to Zoom |
Figure 2: CT showing mass and pleural effusion |
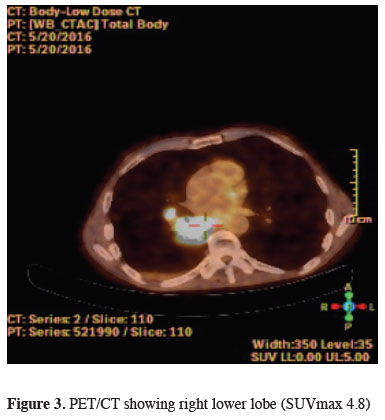
In the patient"s positron emission tomography, 4 x 3 cm mass in the right lower lobe (SUVmax 4.8) and 3 cm pleural effusion (no involvement) was detected (Figure 3).
 Click Here to Zoom |
Figure 3: PET/CT showing right lower lobe (SUVmax 4.8) |
Squamous cell lung cancer has been reported as a result of bronchoscopic biopsy. USG guided thoracentesis was performed for staging on right hemothorax and 4 cc pleural fluid was taken and sampled without any complications.
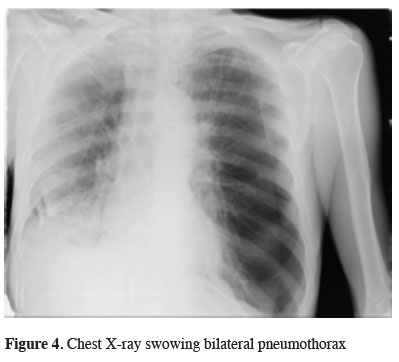
The chest x-ray which was taken due to the patient"s complaint of shortness of breath, which started about 10 minutes after the procedure, demonstrated a partial basal pneumothorax on the right and subtotal pneumothorax on the left side (Figure 4).
 Click Here to Zoom |
Figure 4: Chest X-ray swowing bilateral pneumothorax |
Bilateral tube thoracostomy was performed and the patient rapidly returned to normal. Thereafter, right and left tube thoracostomy were terminated, respectively.
Discussion
Pneumothorax may be divided in three groups as spontaneous, traumatic and iatrogenic. Iatrogenic pneumothorax may develop due to causes such as thoracentesis, percutaneous lung biopsy, bronchoscopy, subclavian vein catheterization, endotracheal intubation, high-pressure ventilation, laparoscopy, and supraclavicular and infraclavicular axillary nerve blocks [5].Aleman et al. reported that in 370 case studies, pneumothorax rate was 4% and asymptomatic pneumothorax rate was about 1% in all patients who underwent PA chest X-ray after thoracentesis [3]. In a similar study involving 199 cases reported by Peterson et al., 251 invasive operations were performed and the risk of pneumothorax was 6.5%, as there was no risk, incidental pneumothorax was detected in 3 (2.3%) patients who had not undergone chest X-ray [4]. In 134 invasive operations, Gerardi et al. reported a total pneumothorax risk as 7.5% [7]. Furthermore, Peterson et al. noted that USG-associated thoracentesis is a risk factor for pleural biopsy.
USG should be used in patients suspected to be on high risk of pneumothorax after thoracentesis [4]. In 2016, John et al. investigated in which hospitals the iatrogenic pneumothorax cases were seen more frequently. According to the results obtained from this study, iatrogenic pneumothorax cases in education hospitals are higher than non-education hospitals. In addition, iatrogenic pneumothorax cases are more frequent in hospitals where thoracentesis is frequently performed. Therefore, chest x-rays should routinely be requested in these two hospitals where iatrogenic pneumothorax cases are common [8]. In the literature chest X-ray is not generally recommended routinely after thoracentesis however in our clinic, although the rate of complications that may develop after thoracentesis is low, chest X-ray is requested as a routine procedure. We strongly recommend radiologic control not to miss such a very rare complication; contralateral pneumothorax.
Declaration of conflicting interests
The author declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The author received no financial support.
Reference
1) Aleman C, Alegre J, Armadans L. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107: 340-3.
2) Light WR. Pleural Diseases. 3rd ed. 1995: 316-21.
3) Peterson WG, Zimmermann R. Limited utility of chest radiograph after thoracentesis. Chest 2000; 117: 1038-42.
4) Fry AW, Paape K. Pneumothorax. In: Shields TW, LoCicero III, Ponn RB, General Thoracic Surgery. 5th ed. Philadelphia: Lippincott Williams & Wilkins. 2000; 675-85.
5) Fayman MS. Air drainage: an essential technique for preventing breast augmentation-related pneumothorax. Aesthetic Plast Surg 2007; 31:19-22.
6) Wax DB, Leibowitz AB. Radiologic assessment of potential sites for needle decompression of a tension pneumothorax. Anesth Analg 2007; 105:1385-8.






