Summary
Background: In this study, the age and cardiac risk factors on mortality, and complications after surgical treatment in lung cancer patients were investigated.Materials and Methods: Between January 2014 and November 2016, 485 patients who underwent lung resections for lung cancer in our clinic, were retrospectively evaluated. Under the age of 70, 412 patients and over 70, 73 patients were grouped as group 1 and 2.
Results: Lobectomy was performed in 333 patients (80.8%), pneumonectomy in 43 (10.4%), bilobectomy in 30 (0.7%), segmentectomy in 3 (0.7%) and sleeve lobectomy in 3 (0.7%) in group 1; lobectomy in 65 (89%), pneumonectomy in 4 (5.4%), and sleeve lobectomy in 4 (5.4%) in group 2. Surgical mortality was observed in 5 patients (1.2%) in group 1 and 6 patients (8.2%) in group 2. Complications were observed in 99 patients (24%) in group 1 and 23 patients (17%) in group 2. The mean expected mortality with cardiac risk score in group 1 was 1.24 and 3.5 in group 2. The expected mortality with cardiac risk score and complication rates were tabulated as low, moderate, high and there was no statistically significant difference between score and complication (p = 0.551).
Conclusions: According to our study, there were no significant differences in terms of complications, morbidity, mortality and survival rates. There was only a relative increase in surgical mortality over the age of 70, so patients should be carefully treated during and after the surgery. Surgery should be the first treatment to perform in selected patients with lung cancer over the age of 70.
Introduction
In recent years, there has been a rapid increase in population over 70 years in our country. The rapid growth of the geriatric population causes the health system to face new problems. The elevated number of the 70-year-old population increases the proportion of patients with lung cancer who underwent surgery in thoracic surgery clinics, and the risk of mortality and complications due to surgical procedures is high [1,2]. By this time in a very small number of studies were focused on patients with operable lung cancer older than 70 years of age, especially those with advanced disease. There are only a few geriatric patient-specific studies for treatment in early stages.In this study, we evaluated the results of surgical resection of lung cancer patients and investigated the effects of preoperative cardiac risk factors on mortality and complications in patients over 70 years of age.
Methods
We retrospectively evaluated 514 patients who were referred to our clinic for surgical treatment between January 2014 and November 2016, diagnosed with lung cancer. In preoperative period; total blood counts, biochemistries, electrocardiographs (ECG) were performed. Echocardiographs were performed with cardiology consultations to the patients who either had pathologic findings on ECG or were over 50 years old. Patients with diabetes and hypertension were consulted for internal medicine and the patients were operated after blood glucose and blood pressure regulations. Also all of our patients’ the expected mortality (logistic score) was determined with cardiac risk score (http://www.euroscore.org/calc.html) (Table 1).Table 1: Risk factors, definitions and weights (score).
The patients with a forced expiratory volume in 1 second (FEV1) value of over 2 liters in the pulmonary function tests were operated without further pulmonary evaluation while carbon monoxide diffusion capacity (DLCO), perfusion scintigraphy, oxygen consumption capacity or exercise tests (6 minutes walk, test) were applied the other patients. If the estimated postoperative FEV1 was below 800 mL and the oxygen consumption capacity was below 15 mL/kg/min, our patients had not undergone surgery.
Patients with mediastinal lymph node involvement who were determined to be able to undergo surgical treatment were first underwent endobronchial ultrasound-guided transbronchial needle aspiration and / or mediastinoscopy. Patients with proven mediastinal metastasis were referred to the oncology clinic for adjuvant or neoadjuvant therapy. Patients without mediastinal metastasis or who had neoadjuvant therapy treated with anatomical lung resection and systematic mediastinal lymph node dissection. Ten patients who were diagnosed with lymph node metastasis by mediastinoscopy, 3 patients who were only explored with thoracotomy, and 16 patients who underwent surgery after neoadjuvant treatment were excluded from the study.
The remaining 485 patients (88 females, 397 males, mean age 59.7 years, range 9 to 82 years) composed our study group. Air leaks that lasted more than 7 days were considered as a prolonged air leak, and the deaths within 30 days after the surgery were considered as surgical mortality. Group 1 was consisted of 412 patients under the age of 70, and group 2 was consisted of 73 patients over 70 years of age.
Statistical analysis
Parametric methods were used for analysis of variables with normal distribution, and non-parametric methods were used for analysis of variables having no normal distribution. Independent sample test and Mann-Whitney U tests were used in comparison of groups among independent groups. Pearson Chi-Square and Fisher’s Exact tests were used to compare categorical data. Kaplan-Meier test was used for survival analysis. Quantitative data were tabulated with mean ± SD values, and categorical data with and percent values. The data were analyzed at 95% confidence level, P values less than 0.05 are considered significant. The Statistical Package for the Social Sciences (SPSS) 21 program was used for statistical analysis. Among the groups, complications, early mortality, hospital stay, survival rates were compared.
Results
In patient groups; smoking histories were found in 350 (84%) patients in group 1 and in 50 (68%) patients in group 2. Lesion locations of the patients in group 1 were: 231 (56%) in the right lung, 181 (44%) in the left lung and in group 2: 39 (53%) in the right lung, 34 (47%) in the left lung. Lobectomy was performed in 333 patients (80.8%), pneumonectomy in 43 patients (10.4%), bilobectomy in 30 patients (7.2%), segmentectomy in 3 patients (0.7%) and sleeve lobectomy in 3 patients in group 1. Lobectomy in 65 patients (89%), pneumonectomy in 4 patients (5.4%), and sleeve lobectomy were performed in 4 patients (5.4%) in group 2 (Table 2).Table 2: Operation procedure, lesion locations and smok-ing histories of our patient.
When postoperative histopathological results were analyzed, the most common tumor cell type was adenocarcinoma (45% and 52%) in both groups, while squamous cell carcinoma cell types were seen 32% in both groups.
Five of the patients in group 1 had mortalities (1.2 %), while in group 2 mortalities were found in 6 patients (8.2%). The most common causes of mortality were with the cardiac origin. In group 1, myocardial infarction was seen in 3 patients, and renal insufficiency in 2; in group 2, 3 of the patients had developed myocardial infarction, renal insufficiency was seen in 2 patients and bronchopleural fistula due to empyema and respiratory insufficiency in 1. 116 complications occurred in 99 patients (24%) in group 1 and 17 (23.3%) in group 2. The most common complication was the failure of the expansion in the lung after surgery in 56 patients [group 1 (n = 50) + group 2 (n = 6)]. Other frequent complications were prolonged air leak in 20 patients [group 1 (n = 18) + group 2 (n = 2)] and infection of operation wound in 14 patients [group 1 (n = 11) + group 2].
The mean hospital stay was 15 days (range 4-99) in group 1, and 15.6 days (range 5-46) in group 2. There was no statistically significant difference between them (p > 0.05). In both groups, the mean length of hospital stay was 14.3 days when the patients with postoperative complications were excluded. The mean duration of hospitalization for the patients with postoperative complications was 18 days.
The expected mortality (logistic score) with cardiac risk score of 412 patients in group 1 was 1.24 and the score of the seventy three patients in group 2 was 3.5. The expected mortality with cardiac risk score and complication rates were tabulated as low, moderate, high and there was no statistically significant difference between score and complication p = 0.551 (Table 3).
Table 3:The expected mortality with cardiac risk score and complication rates.
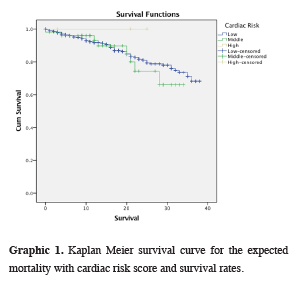
The expected mortality with cardiac risk score and survival rates were shown on the table 5 and a Kaplan Meier survival curve was drawn by Log-rank method (Graphic 1). There was a statistically significant difference in mortality between low cardiac risk and moderate cardiac risk p = 0.028.
 Click Here to Zoom |
Graphic 1: Kaplan Meier survival curve for the expected mortality with cardiac risk score and survival rates. |
Table 4: Mean survival rates of our patients.
Table 5: The expected mortality with cardiac risk score and survival rates.
Discussion
Lung cancer is the leading cause of cancer-related deaths worldwide, and non-small cell lung cancer (NSCLC) constitutes more than 80% of lung cancers [3]. Surgery is the best-recommended treatment for patients with early stage lung cancer [4].The median age of newly diagnosed NSCLC in the USA is 68 years, and more than 40% of newly diagnosed patients are over 70 years of age and 14% are over 80 years old [5]. Given the increasingly aging world population, it is clear that we will face more patients with NSCLC in the coming years and therefore it is frequently encountered with heart diseases, chronic obstructive pulmonary diseases, diabetes, renal dysfunctions and other medical problems. For this reason, the choice of treatment for patients over 70 years of age is becoming increasingly important [6]. In our study, 73 (15%) of 485 patients who were older than 70 years of age were diagnosed as lung cancer and underwent surgical treatment. It was found that, the number of patients in this age group increased when compared with the rates in the past years. However, compared to other studies, it is possible that the low numbers of elderly patients with lung cancer treated surgically in our country, might be due to the high population of young people, the desire of elderly patients and their relatives to refuse surgery, and the sensitivity of surgeons to do the surgery.
In our study, no patients were evaluated as inoperable due to advanced age. Lymph node dissection was performed with complete lung resection for all patients. None of the patients underwent limited lung resection. From the point of view of surgery, pneumonectomy was applied to 10% of patients under the age of 70, and to 5% of patients over 70 years of age. This difference may be due to limited respiratory capacity in patients over 70 years of age and consequently the decision to perform pneumonectomy was taken cautiously. When planning surgical treatment in elderly patients, we should consider the following: the mortality rate of the surgeon to be performed should be acceptable, and should not exceed 4% for lobectomy and 8% for pneumonectomy [7,8]. Dalwadi et al. [9] stated that their data with elderly patients, stereotactic body radiation therapy (SBRT) replaced surgery as the most used modality for early stage NSCLC. Selecting elderly patients with comorbidities appropriately, surgical outcomes declined with age but the SBRT outcomes did not.
In the literature, after a lung resection, associated pulmonary complications such as prolonged air leak, pneumonia, pulmonary embolism, respiratory failure, and bronchopleural fistula have been reported more frequently [4,10-12]. Among cardiac complications, rhythm disorders were reported between 3.8% and 37% [13]. Toker et al. reported that cardiac complication rates were 20% higher than the other series, and age, sex, and past myocardial infarction after pulmonary surgery have not been associated with major complications and mortality [14]. In another study, no significant relationship was found between age and postoperative pneumonia [2]. Another study reported that cardiopulmonary complications were more common in the elderly than in younger patients, but the only difference for risk of death between the two groups was reported as pulmonary complication. Therefore, elderly patients should be evaluated in detail before surgery [2,4]. Some authors have advocated the preoperative evaluation of patients over the age of 60, the need for cardiologic examination, whether symptomatic or asymptomatic [2]. In our study, we performed ECG with cardiology consultations and echocardiography examination, pulmonary function tests or advanced tests for all patients over 50 years old, and after evaluating the patient was appropriate, anatomical lung resections were performed. The complication rate was 23.3% in group 2 and 24% in group 1, but this difference was not significant between the two groups. 116 complications occurred in 99 patients in group 1 and 17 in group 2. The most common complication was postoperative expansion failure of the lung (n = 56). Other frequent complications were prolonged air leak in 20 patients and infection of operation wound in 14 patients.
The morbidity rate after anatomical pulmonary surgery was reported to be about 30%, after pneumonectomy was between 15% and 75%, and was found to be higher than in other lung resections; cardiac complications, most often rhythm disorders were reported between 3.8% to 37%, and the incidence of arrhythmia in elderly patients were reported 10,7% in the series [10,14]. The cause of arrhythmia is not exactly known. The possible causes are mediastinal shift, hypoxemia, and abnormal blood pH. However, the well-known reputation is, arrhythmia formation occurs in elderly patients, after a coronary artery disease and extended resections, especially after intrapericardial pulmonary vascular ligation [10]. Risk of life-threatening pulmonary embolism following surgery includes increased physiological changes in the cardiovascular system and respiratory system related to the age, diabetes, kidney and liver diseases [7,15–17]. For these reasons, a number of studies have been carried out to explain whether morbidity and mortality rates after geriatric patients treated with surgery for lung cancer are affected by age.
In surgical treatment, the known mortality rate for pneumonectomy is between 3-12%. The mortality rate for lobectomy is 1-4% and it is half of the mortality rate of pneumonectomy [10]. Roxburgh et al. [11] reported that mortality was higher in older patients after pneumonectomy, but the difference was not significant. Dyszkiewicz et al. [18] reported mortality of pneumonectomy in elderly patients was 17% and mortality was not detected in the same group with lobectomy or wedge resection. The mortality rates for patients over 70 years of age were reported as 2.4% for lobectomy, 12.5% for pneumonectomy, and 4.2% for lobectomy in patients with lung cancer over 80 years old [10]. Rostad and Mark [19] found that the mortality rate doubled between the ages of 70-79. Different results have been reported regarding postoperative mortality and morbidity rates in elderly patients in general. Many authors have stated that the risk is higher in older people, but recent studies have shown that the mortality rate is acceptable [2] Careful patient selection is very important to reduce the expected higher mortality and morbidity rate. Suemitsu et al. [20] have indicated that surgical treatment is useful for selected elderly patients who may be able to tolerate surgery and achieve complete resection [20,21]. In our study, surgical mortality of group 1 was found 1.2% (n = 5), while in group 2.2% (n = 6). Mortality rates are better in group 1 when compared with the literature, and in group 2 are in the same range. In this case, there should be a more experienced team in group 2 and more careful operations should be done.
The survival rate should be increased after surgery and the quality of life should not deteriorate after surgery [8]. Onaitis et al. [22] had stated that, thoracoscopic approach is associated with improved long-term survival in elderly patients in comparison to thoracotomy, although it is difficult to explain the advantages of long-term survival. Procedure choice and approach choice is complex and cannot be easily decided. In our study, with carefully selected patients over 70 years of age with NSCLC, there was no significant increase in complication rates and survival rates.
Evaluation with the cardiac risk score showed that, age-related score were found to be higher in the group with over 70 years of age, but there was no statistically significant difference between the groups in terms of survival, complications and other parameters except early mortality. As the number of these studies increase, the age factor for cardiac risk scoring can be reevaluated. Due to the increase in early mortality in patients over 70 years of age, careful follow-up and treatment of the patients during and after the operation should be performed and coordinated multidisciplinary and necessary treatments should be given to the patients. New studies has begun for geriatric patients for the last 10 years, but in these studies the majority of patients are in the early 70’s and the number of patients over 80 is low [23,24]. According to the new age groups of the World Health Organization, between the ages of 66-79 were determined as middle ages and between 80-99 as elderly. For that reason, more studies should be done in the group of patients over 70 years old.
As a conclusion, in the presence of early stage pulmonary malignancy, surgical treatment should be considered primarily in patients over 70 years of age. Being 70 years or older should not be a single contraindication for surgery, or indications for limited resection alone. We believe that this age group of patients may also be treated surgically with an acceptable risk level.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Reference
1) Demirci NY, Ulger S, Yılmaz U, Aydogdu K, Yilmaz, Erdogan Y. Radical Oncological Surgery and Adjuvan Therapy in Non-Small Cell Lung Cancer Patients over 70 years of Age. Asian Pacific Journal of Cancer Prevention 2015; 16: 4711-4.
2) Melek H, Medetoğlu B, Demir A, Kara V, Dinçer Sİ. Mortality and morbidity after surgical treatment in elderly patients with non-small cell lung cancer: the role of age. Turkish J Thoracic Cardiovasc Surg 2011; 19: 586-92.
3) Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006; 24: 4539-44.
4) Spaggiari L, Scanagatta P. Surgery of non-small cell lung cancer in the elderly. Current opinion in oncology 2007; 19: 84-91.
5) Owonikoko TK, Ragin CC, Belani CP, Oton AB, Gooding WE, Taioli E, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. Journal of clinical oncology 2007; 25: 5570-7.
6) Simmonds MA. Cancer statistics, 2003: further decrease in mortality rate, increase in persons living with cancer. CA: a cancer journal for clinicians 2003; 53: 4.
7) British Thoracic Society; Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 2001; 56: 89-108.
8) Okamoto T, Maruyama R, Shoji F, Ikeda J, Miyamoto T, Nakamura T, et al. Clinical patterns and treatment outcome of elderly patients in clinical stage IB/II non-small cell lung cancer. J Surg Oncol 2004; 87: 134-8.
9) Dalwadi SM, Szeja SS, Bernicker EH, Butler EB, Teh BS, Farach AM. Practice patterns and outcomes in elderly stage I non-small-cell lung cancer: a 2004 to 2012 SEER analysis. Clin Lung Cancer 2018; 19: 269-76.
10) Haithcock BE, Richard HF. Complications of Pulmonary Resection. Shields MD, Thomas W. LoCicero J; Reed CE. Feins RH. General Thoracic Surgery. 7th Edition 2009; 558.
11) Roxburgh JC, Thompson J, Goldstraw P. Hospital mortality and longterm survival after pulmonary resection in the elderly. Ann Thorac Surg 1991; 51: 800-3.
12) Voltolini L, Rapicetta C, Ligabue T, Luzzi L, Scala V, Gotti G. Short- and long-term results of lung resection for cancer in octogenarians. Asian Cardiovasc Thorac Ann 2009;17: 147-52.
13) Asamura H, Naruke T, Tsuchiya R, Goya T, Kondo H, Suemasu K. What are the risk factors for arrhythmias after thoracic operations? A retrospective multivariate analysis of 267 consecutive thoracic operations. J Thorac Cardiovasc Surg. 1993; 106:1104-10.
14) Toker A, Solak O, Günlüoğlu Z, Kocatürk C, Dinçer İ, Kullep M, et al. Yaşlı hastalarda akciğer kanseri cerrahi tedavisi. Solunum 2001; 3: 9-13.
15) Thistlethwaite PA, Madani M, Jamieson SW. Chronic pulmonary emboli. In: Shields TW, Cicero JL, Reed CE, Feins RH, editors. General thoracic surgery. Philadelphia: Lippincott Williams &Wilkins; 2009. pp. 1067-76.
16) Satoh Y, Okumura S, Nakagawa K, Horiike A, Ohyanagi F, Nishio M, et al, Postoperative ischemic change in bronchial stumps after primary lung cancer resection. Eur J Cardiothorac Surg 2006; 30: 172-6.
17) Ciriaco P, Casiraghi M, Melloni G, Carretta A, Libretti L, Augello G, et al. Pulmonary resection for non-smallcell lung cancer in patient son hemodialysis: Clinical outcome and long-term results. World J Surg 2005; 29: 1516-9.
18) Dyszkiewicz W, Pawlak K, Gasiorowski L. Early post-pneumonectomy complications in the elderly. Eur J Cardiothorac Surg 2000; 17: 246-50.
19) Rostad H, Strand TE, Naalsund A, Talleraas O, Norstein J. Lung cancer surgery: the first 60 days (A population-based study) . Eur J Cardiothorac Surg. 2006; 29: 824-8.
20) Suemitsu R, Yamaguchi M, Takeo S, Ondo K, Ueda H, Yoshino I, et al. Favorable surgical results for patients with nonsmall cell lung cancer over 80 years old: a multicenter survey. Ann Thorac Cardiovasc Surg 2008; 14: 154-60.
21) Jaklitsch MT, Mery CM, Audisio RA. The use of surgery to treat lung cancer in elderly patients. Lancet Oncol 2003; 4: 463-71.
22) Onaitis MW, Furnary AP, Kosinski AS, Kim S, Boffa D, Cowper P, et al, Prediction of long-term survival following lung cancer surgery for elderly patients in the Society of Thoracic Surgeons General Thoracic Surgery Database. Ann Thor Surg 2018; 105: 309-16.






