2Department of Thoracic Surgery, Atatürk Chest Diseases and Thoracic Surgery Training and Research Hospital, Ankara, Turkey
3Department of Pediatrics, Necip Fazıl City Hospital, Kahramanmaraş, Turkey
4Department of Pulmonary Medicine, School of Medicine, Kahramanmaraş Sütçü İmam University, Kahramanmaraş, Turkey DOI : 10.26663/cts.2016.0005
Summary
Background: The main subtypes of chest wall deformities are pectus excavatum (PE) and pectus carinatum (PC). Although these are not life threatening diseases, some functional or physico-social disabilities due to the abnormal chest wall structure require treatment. The main stem of the treatment is surgical correction. Since the beginning of the 20th century various surgical techniques were introduced. In the last few decades some minimally invasive surgical techniques were also described. In the English literature many reports suggest that the vast majority of these abnormalities are PE. But in most of the reports patient groups consist of hospital admissions. The real frequency of this group of disease is controversial.Materials and Methods: In this report, a wide field study was designed to find the real frequency of the chest wall diseases. Total of 25117 children between 6-11 years of age were visited in the elementary schools of Kahramanmaraş. The team members were a thoracic surgeon, a pulmonologist and a pediatrician.
Results: A careful physical examination revealed that a total of 255 patients had different degrees of chest wall deformity. We found that PC (90.58%) was the most frequent type of deformity in contrast with the previous reports suggesting PE as the most frequent type. In our results only 5.49% of the patients had PE and 3.93% of the patients had mixed type PE+PC. The most frequent ECG abnormality seen in 49 cases (19% of cases with deformity) was a negative T wave on V1 derivation and a biphasic T wave configuration on V1-V2 derivations. 8 patients (3.13%) had concomitant scoliosis and 6 patients (%2.35) had different degrees of cardiomegaly.
Conclusions: The real frequency of chest wall deformities is an enigma. When PE patients themselves or their family notice the abnormality, they may consider it as a big health problem and admit to a healthcare unit. But in contrast, PC patients mostly do not care about this disorder until adolescence, so admission rate is less then PE. We suggest that the real frequency of the chest wall deformities may be found only by widespread field studies. Moreover, we found more accompanying cardiac disorders in PC group, and this type of screening may allow early diagnosis of some cardiac diseases.
Introduction
Chest wall deformities are a group of diseases changing the symmetric structure of the chest wall [1]. Most of these deformities do neither threat life nor lead a functional disorder. The classification by Willital offers 11 sub-branches for chest wall deformities which consists of pectus excavatum (PE) (4 subtypes), pectus carinatum (PC) (4 subtypes), mixed type, chest wall aplasia/hypoplasia and sternal cleft [2] (Table 1). The most frequent type of chest wall deformities is PE which consists of nearly 90% of the whole group [3].Table 1: Willital classification for congenital chest wall deformities
In our study a definite population of otherwise healthy school children is screened to define the prevalence of chest wall deformities, to compare these data with the literatüre and children with deformity were invited to our hospital to make additional tests and the definitive treatment.
Methods
An ethics committee approval and required permission was obtained from the Turkish Ministry of Health Kahramanmaraş Health Administration. A screening team consisting of a thoracic surgeon, a pulmonologist and a pediatrician was established. Screening was performed by visiting children in predefined elementary schools. The children identified with a chest wall deformity, were invited to hospital with his/her family. This group had undergone additional tests to clarify if a surgical treatment was indicated. Results were classified and compared with recent studies.Results
A total of 25117 students between 6-11 years (mean 8.54) were included. The 56.9% of children were girls and 43.1% were boys. A total of 255 (1, 01%) individuals with a chest wall deformity were invited to hospital for further evaluation. These were classified according to the type of deformity and additional tests were performed. Results were compared with recent studies.231 of 255 cases (90.58%) had PC, 14 had PE (5.49%) and 10 had mixed PE+PC deformity (Table 2).
Table 2: Summary of the results
128 cases had an abnormal electrocardiogram (ECG) record. The most frequent ECG abnormality seen in 49 cases (19% of cases with deformity) was a negative T wave on V1 derivation and a biphasic T wave configuration on V1-V2 derivations. 8 patients (3.13%) had concomitant scoliosis. 6 patients (2.35%) had different degrees of cardiomegaly (Table 3).
Table 3: Distribution of accompanying pathologies
Discussion
A morphologic classification which is defined as "Willital Classification", is generally used for classifying chest wall deformities [2]. When abnormalities such as Pentalogy of Cantrell, sternal cleft and Poland"s Syndrome which make only 3.4% are excluded, chest wall deformity mostly refers to pectus deformities and especially pectus excavatum (PE) with a frequency of more than 90% in the literatüre [4].
Pectus Excavatum
Pektus excavatum, or "Funnel chest", is the most frequent type of chest wall deformity. It is the depression of the anterior chest wall in a symmetric or asymmetric manner due to abnormal growth of sternum and / or costal cartilage. In a report by Robiscek and colleagues, it is stated that this deformity is seen in 0.32% of all births by 9 times more in males [5]. Matos et al. stated that while the percentage reported was changing between different authors, its incidence was 0.1% by a male/female ratio of 3.3 [6]. Nuss et al. reported male / female ratio as 4 in their series of 148 cases [7]. Yüksel et al. reported that PE was seen 1 per 300-400 live births [8]. Brochhausen et al. reported an incidence of 0.1-0.8% and a male/female ratio between 2 and 9 [4].
In this study, we have found that PE consists only in 5,49% of all chest wall deformities, and its ratio in general population was 0.05%. Male / female ratio was found to be equal. When compared to previous two other studies made in Turkey, our data was similar with the report by Yücesan et al. who had found PC incidence higher [9]. But the other report by Esme et al. a states a higher PE incidence [10]. Groetsky et al. also reported a higher PC incidence than PE in Argentina [11].
Robiscek et al. stated that PE was a recessively transmitting disease, but Jarowski et al. reported that although 40% of the cases had a family history, it was not possible to introduce a strict relation to genetics [5,12]. Wu et al. reported that the disease was a recessive genetic disease and 40% of the cases had a family history of chest wall deformity, besides they defined a mutation on GAL3ST4 gene [13]. In the etiopathogenesis of PE, intrauterine compression, rickets, pulmonary restriction and insufficiency in osteogenesis or chondrogenesis were the considered theories [14]. In our study, a positive family history was present in only 2 PE cases (14.2%).
According to Robiscek et al. a majority of patients suffered from decreased effort capacity, decreased mobility, chest pain, palpitation and recurrent pulmonary infections [5]. Matos et al. reported that majority of the cases were asymptomatic until adolescence but suffered from decrease in effort capacity and dyspnea [6]. Nuss et al. stated that exercise intolerance, recurrent pulmonary infections and symptoms of asthma were present in these patients [7]. Johnson et al. defined the most frequent symptoms in PE as decreased capacity of exercise and chest pain [15].
On the other hand Oezcan et al. evaluated 18 patients who had a pectus index greater than 3 with echocardiography and cardiac magnetic resonance imaging, and found the most frequent concomitant cardiac pathologies as pericardial effusion, a left-sided cardiac shift and tricuspid valve prolapse [6]. In our study the most frequent symptoms in PE were cosmetic discomfort in 5 (35.7%), chest pain in 2 (14.8%) and dyspnea in 2 (14.8%) patients.
Nuss et al. found 5 Marfan Syndrome and 2 Ehlers Danlos Syndrome in a series of 127 PE patients [7]. Also Robiscek et al. reported that scoliosis, mitral valve disease and Marfan Syndrome might accompany PE [5]. Töpper et al. showed an increase in ejection fraction of the right ventricle following the surgical correction of PE in a prospective study [17]. In our study one patient had an accompanying scoliosis and 3 of the patients had negative T wave on anterior chest derivations on ECG.
In physical examination, the typical pectus posture is seen, which is, thin and tall children with a symmetric or asymmetric sternal depression, depressed shoulders, an increase in thoracic kyphosis and a relative bump on the abdomen. Accompanying pathologies such as scoliosis, asymmetric scapula, shift of cardiac apex beat, cardiac murmur, tachycardia, and arrhythmia may be present [4].
The degree of depression, location of the mediastinal organs and accompanying scoliosis are evaluated by chest x-ray and computed tomography. The pectus index defined by Haller et al. in 1987 is the ratio of transverse to antero-posterior dimension of chest wall on the most distinct depression. It is used for determining the degree of the deformity and surgical indication in PE. According to this index, a value lower than 2.56 is defined as normal, while higher than 3.25 suggests surgical correction. This index has replaced the old index defined by Welch in 1980, which suggested estimating the distance between vertebra and sternum in lateral chest x-ray [18] (Figure 1).
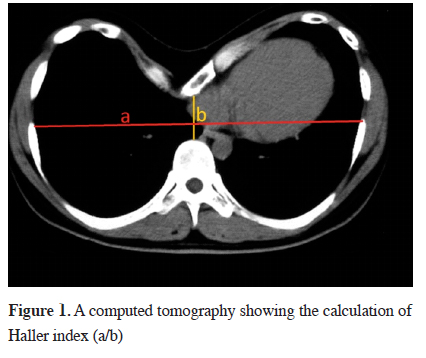 Click Here to Zoom |
Figure 1: A computed tomography showing the calculation of Haller index (a/b) |
The management of PE is on a wide range from observation by follow up to surgical treatment or non-surgical treatments like silicone or vacuum therapy. The minor deformity which doesn"t lead to physiologic or psychological problems may be observed and followed up until the adolescence. Jarozewski and colleagues have defined surgical indications in PE [12] (Table 4).
Table 4: Criteria for surgical referral
Zang et al. defined surgical indications for pectus correction in a series of 639 cases as, Haller index bigger then 3.25, restrictive or obstructive pattern in respiratory function tests, valve prolapse on ECG or echocardiography, presence of a progressive and symptomatic deformity, physicosocial problems due to abnormal body structure, recurrence in patients underwent previous surgery [19]. Historically the first successful surgery for PE was reported by Meyer in 1911 followed by reports defining different techniques until the development of minimally invasive techniques we use today.
The milestone in PE surgery is the report by Ravitch describing his new technique and giving the results of 8 cases. In subject report Ravitch had classified surgical indications in main three topics; cosmetic, orthopedic and physiologic. His technique was consisting of a vertical incision on sternum, subperichondrial total resection of deformed cartilages, the resection of intercostal bands, resection of the xiphoid process, sternal osteotomy and a substernal transverse Kirschner wire placement between the remaining costae [20]. This technique was applied with some modifications in the following years. The sternal turnover technique defined by Wada in 1960 was used until 1970 on 271 cases but it was then left due to high complication rate [21].
In 1978, Robiscek have modified the technique of Ravitch by using a marlex mesh. In this technique after the mobilization of the sternum a transverse osteotomy is made, parasternal costal cartilages were resected and then a marlex mesh is laid behind the sternum between the ends of the resected costa for chest wall stabilization [22]. Also Robiscek et al. determined the surgical indications as: i) This surgery may be performed in any age, ii) Morbidity must be kept in minimal and must not be prolonged, iii) No mortality is allowed, iv) The anatomic result must be perfect [5].
Gurkok et al. defined a new technique for PE correction which an absorbable plaque is placed with polymer screws on the point of osteotomy but the main disadvantage of this technique is high costs [23,24].
The minimally invasive technique in PE correction was defined by Donald Nuss in 1998 on 50 patients. This technique is called by his name as "Nuss Procedure" [7]. In this procedure, while the cosmetic results, pain control and the days of hospital stay is more favorable then open surgical techniques, some serious complications such as pneumothorax, pleural effusion, cardiac injury and dislocation of the bar may be seen [25,26]. In our study, surgical PE correction with Nuss procedure was offered to 9 patients who had cosmetic problems and physical symptoms.
Pectus Carinatum
According to the majority of the literatüre, it is a more rare deformity when compared with PE with a prevalence of 0.06-0.09% [27]. But, Goretsky et al. reported that it was seen more than PE in Argentina [11]. It is more frequent in males [27]. Robiscek and colleagues reported male / female ratio as 4 in 2010 [28]. Fonkalsrud reported a 6 fold male incidence [29]. The etiology is not clear like PE, but the most common view is the abnormal development of costal cartilages [30]. In our study, in contrast with the literature, the vast majority of the deformities were PC (90.5%). We think that the main cause of this difference is because the notification of PE is easier by the children themselves or by their families and it is thought to be a severe condition, so the hospital admissions are much more than PC. As a result, the hospital incidence of PE is greater than PC.
PC is also classified as symmetric or asymmetric due to the place of the sternal protrusion. As PE, Marfan syndrome, homocystinuria, Prune belly syndrome, Morquio syndrome and mitral valve prolapse may accompany PC, too [28]. Coelho et al. reported 16.4% accompanying asthma and chronic bronchitis and in almost whole patients kyphosis in different degrees [27]. Robiscek et al. reported that one third of the patients had a family history while Park et al. reported that 25% of patients said that a chest wall deformity was present in the family [28,30]. In our study the incidence of PC was 0.91% of all the study population. Male / female ratio was 3.2 which was similar to the literature. But when family history is questioned, a positive history ratio of 3.03% was found and this was really low when compared to the literature. We think the cause of this low ratio may be due to mild deformities which are not defined as an abnormality by the people.
Coelho et al. reported that palpitation and dyspnea triggered by effort were the symptoms of PC. Also these patients avoided activities such swimming or other sports which might expose their deformity [27]. Fonkalsrud reported that symptoms in PC rarely came out in early childhood, mostly they began in adolescence and the most frequent ones were dyspnea, exercise intolerance and exersize associated wheezing. But the most disturbing state is the cosmetic one for an adolescent [29]. In our study the most frequent symptoms were found as dyspnea, palpitation and exercise intolerance, too. In 7 patient with PK (3.03%) accompanying scoliosis and in 6 patients (2.59%) different degrees of cardiomegaly was found.
There is limited experience with using supportive materials and chest compressors for the treatment of PC. For surgical treatment, Robiscek and Ravitch defined costochondral resection and sternotomy technique. In this technique, whether a unilateral deformity is present, it is important to make bilateral cartilage resections for preventing recurrence [11]. In the report by Robiscek in 2011, the below listed points were emphasized in modern PC surgery. The technique must be easy and feasible for fast application, must not require permanent foreign material in the body, must not lead to severe complications, must have a perfect cosmetic and functional result, must not require long and frequent postoperative control and must not require re-intervention.
The minimally invasive technique was defined by Abramson in 2008. In his report 40 cases were evaluated in terms of effectiveness. In this technique, the bar was introduced subcutaneously through bilateral incisions on the chest wall.
Minimally invasive technique in PC is used in the chondrogladiolar subtype. According to this report, the advantages of this minimally invasive technique are listed as [31], lack of incisions in the anterior thoracic region, avoiding scars and the presence of keloid formation, short surgery time and minimal blood loss, short hospitalization and recovery time, improvements in the thoracic contour because of correction of the protrusion and widening of base of both hemithorax without losing elasticity in the thoracic cage, very good long term results without recurrences.
The metal bar designed by Yuksel et al. was used in 18 cases with the technique defined by Abramson and they had very satisfactory results[32]. In our study, considering the degree of the deformity and the present symptoms 47 patients (20.3%) were offered a surgical correction.
In conclusion, we think that for calculating the real incidence of chest wall deformities in the population, widespread screening studies must be designed at schools, military camps and colleges instead of counting hospital admissions. In our study, in contrast with the literature, 90% of the deformities were found to be PC. The "family history" definition is a subjective data and families also must be included in screening programs. Moreover, we found more accompanying cardiac disorder in PC group, and this type of screening may allow early diagnosis of some cardiac diseases.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Reference
1) Anger J, Francisco R, Alcalde V, de Campos JR. The use of soft silicone solid implant molded intraoperative for pectus excavatum surgical repair. Einstein 2014; 12: 186-90.
2) Saxena AK. Pectus excavatum, pectus carinatum and other forms of thoracic deformities. J Indian Assoc Pediatr Surg 2005; 10: 147-57.
3) Kuru P, Cakiroglu A, Er A, Ozbakir H, Cinel AE, Cangut B, et al. Pectus excavatum and pectus carinatum: Associated conditions, family history, and postoperative patient satisfaction. Korean J Thorac Cardiovasc Surg 2016; 49: 29-34.
4) Brochhausena C, Turialb S, Müllera KF, Schmitta VH, Coerdta W, Wihlmc JM, et al. Pectus excavatum: history, hypotheses and treatment options. Interact Cardiovasc Thorac Surg 2012; 14: 801–6.
5) Robicsek F, Watts LT, Fokin AA. Surgical Repair of pectus excavatum and carinatum. Semin Thorac Cardiovasc Surg 2009; 21: 64-75.
6) de Matos AC, Bernardo JE, Fernandes LE, Antunes MJ. Surgery of chest wall deformities. Eur J Cardiothorac Surg 1997; 12: 345-50.
7) Nuss D, Kelly RE, Crotioru DP, Katz ME. A 10 year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg, 1998; 33: 545-52.
8) Yuksel M, Bostanci K, Evman S. Minimally invasive repair after inefficient open surgery for pectus excavatum. Eur J Cardiothorac Surg 2011; 40: 625-9.
9) Yucesan S, Dındar H, Olcay I, Okur H, Kilicaslan S, Ergoren Y, et al. Prevalence of congenital abnormalities in Turkish school children Eur J Epidemiol 1993; 9: 373-80.
10) Esme H, Bukulmez A, Dogru O, Solak O. Prevalence of chest wall deformities in primary school children of Afyon city. Turkish J Thorac Cardiovasc Surg 2006; 14: 34-7.
11) Goretsky MJ, Kelly RE, Jr, Croitoru D, Nuss D. Chest wall anomalies: pectus excavatum and pectus carinatum. Adolesc Med 2004; 15: 455-71.
12) Jaroszewski D, Notrica D, McMahon L, Steidley E, Deschamps C. Current management of pectus excavatum: A review and update of therapy and treatment recommendations. JABFM 2010; 23: 230-39.
13) Wu S, Sun X, Zhu W, Huang Y, Mou L, Liu M, et al. Evidence for GAL3ST4 mutation as the Potential cause of pectus excavatum Cell Res 2012; 22: 1712-5.
14) Tocchioni F,Ghionzoli M, MessineoA, Romagnoli P. Pectus excavatum and heritable disorders of the connective tissue. Pediatric Reports 2013; 5: 58-63.
15) Johnson WR, Fedor D, Singhal S. Systematic review of surgical treatment techniques for adult and pediatric patients with pectus excavatum. J Cardiothorac Surg 2014, 9: 25.
16) Oezcan S, Attenhofer Jost CH, Pfyffer M, Kellenberger C, Jenni R, Binggeli C, et al. Pectus excavatum: echocardiography and cardiac MRI reveal frequent pericardial effusion and right-sided heart anomalies. Eur Heart J Cardiovasc Imaging 2012; 13: 673-9.
17) Töpper A, Polleichtner S, Zagrosek A, Prothmann M, Traber J, Schwenke C, et al. Impact of surgical correction of pectus excavatum on cardiac function: insights on the right ventricle. A cardiovascular magnetic resonance study. Interact CardioVasc Thorac Surg 2016; 22: 38-46.
18) Haller JA, Kramer SS, Lietman SA. Use of CT scans in selection of patients for pectus excavatum surgery: A preliminary report. J Pediatr Surg 1987; 22: 904-6.
19) Zhang DK, Tang JM, Ben XM, Xie L, Zhou HY, Ye X, et al. Surgical correction of 639 pectus excavatum cases via the Nuss Procedure. J Thorac Dis 2015; 7: 1595-605.
20) Ravitch MM. The operative treatment of pectus excavatum. Ann Surg 1949; 129: 429-44.
21) Wada J, Ikeda K , Ishida T, Hasegawa T. Results of 271 funnel chest operations. Ann Thorac Surg 1970; 10: 526-32.
22) Robicsek F. Marlex mesh support for the correction of very severe and recurrent pectus excavatum. Ann Thorac Surg 1978; 26: 80-3.
23) Gurkok S, Genc O, Dakak M, Balkanli K. The use of absorbable material in correction of pectus deformities. Eur J Cardiothorac Surg 2001; 19:711-2.
24) Ozpolat B, Soyal T, Gokaslan G, Tophanelioglu T, Sarigül A,Yücel E. Surgical correction of pectus excavatum and carinatum deformities: evaluation of 30 cases. Turkish J Thorac Cardiovasc Surg 2005; 13: 270-3.
25) Cheng YL, Lin CT, Wang HB, Chang H. Pleural Effusion Complicating after Nuss Procedure for pectus excavatum. Ann Thorac Cardiovasc Surg 2014; 20: 6-11.
26) Sa YJ, Lee J, Jeong JY, Choi M, Park SS, Sim SB, et al. A clinical decision-making model for repeat surgical treatment of pectus Bar displacement: distance measurement after nuss procedure. J Cardiothorac Surg 2016; 11: 16.
27) Coelho S, Guimaraes S. Pectus carinatum. J Bras Pneumol 2007; 33: 463-74.
28) Robicsek F, Watts LT. Pectus carinatum. Thorac Surg Clin 2010; 20: 563-74.
29) Fonkalsrud EW. Pectus Carinatum: The undertreated chest malformation. Asian J Surg 2003; 26: 189-92.
30) Parka CH, Kima TH, Haamb SJ, Lee S. Does overgrowth of costal cartilage cause pectus carinatum? A three-dimensional computed tomography evaluation of rib length and costal cartilage length in patients with asymmetric pectus carinatum. Interact Cardiovasc Thorac Surg 2013; 17: 757-63.






