2Department of Pediatric Endocrinology, Ege University, School of Medicine, Izmir, Turkey DOI : 10.26663/cts.2019.0004
Summary
Background: Although the etiology of primer spontaneous pneumothorax (PSP) is not exactly understood, it has been suggested to be associated with rupture of bulla/bleb formations at the lung apices in thin, tall, young adults. In this study, we aim to evaluate whether delayed puberty is a new etiological factor for PSP.Materials and Methods: A total of 91 patients who were treated for PSP in our clinic between January 2013 and September 2016 and who were scheduled for a follow-up visit by phone call and underwent pubertal-stage assessment were retrospectively analyzed.
Results: Of all patients, 10 (12.1%) were females and 81 (87.9%) were males. The mean age was 25.90 ± 6.83 (range, 17 to 38) years and the mean height was 178.90 ± 7.6 (range, 161 to 197) cm. The mean age at menarche of female patients was 14.20 ± 1.31 (range, 12 to 17) years. The women had a late age at menarche compared to the overall Turkish female population (p < 0.0001). The mean age for the development of facial hairs in the men was 16.63 ± 1.69 (range, 13 to 22) years, showing a later age, compared to the overall Turkish male population (p < 0.0001).
Conclusion: Our study results showed that the patients with pneumothorax had an advanced age at puberty, compared to the normal overall population, and that the pubertal growth spurt occurred within a short period of time. This condition is also suggested to have an effect on the lung maturity and the development of pneumothorax.
Introduction
Spontaneous pneumothorax refers to a condition where there is accumulation of air in the pleural space in the absence of trauma. The condition is termed primary spontaneous pneumothorax (PSP) when there is no underlying pulmonary disease, which is observed in one per 5.8 to 16.7/100000 individuals of the overall population [1]. Although the etiology is not well-understood, it has been suggested to be associated with rupture of bulla/bleb formations at the lung apices in young, tall, and mostly thin adults [2]. Cigarette smoking is thought to play a role in its etiology; however, there is no definite opinion as to the mechanism of action [3]. Mortality is reported to be low; however, it is considered as a disease with a high mortality rate, taking into consideration that it has a recurrence rate of 16 to 52% [1,4].Retrospective evaluation of the structural development and delayed puberty is a condition performed by observing the age at menarche in girls, and growth spurt, and age for facial hair development in boys. The growth rate is known to be small and pubertal spurt is delayed for about 2 to 4 years in cases with delayed puberty, compared to normal pubertal years. During pubertal spurts, the annual growth rate is observed to be lower than that of the normal population; however, the duration of pubertal spurt is prolonged. Growth recorded during pubertal spurts is known to be about 2.9 cm smaller than normal children. Sudden increase in height is expected, since there is a shorter duration of pubertal spurt, and is observed six-fold more in boys than girls [5-7].
Pneumothorax is observed six times more in males with delayed structural development. Besides, it is reported to be shown by conditions closely associated with growth and development such as tall height. Based on this relationship, a history of delayed puberty and late growth should be obtained in the life young adults who present with a diagnosis of pneumothorax. The aim of this study is to bring a new perspective to the etiology of PSP, and to review conditions of growth and development of the cases involved.
Methods
A total of 266 patients who were treated for PSP in our clinic January 2013 and September 2016 were retrospectively evaluated. Patients with syndromic features and those with a history of familial pneumothorax were excluded from the study. The remaining patients were requested by phone to come for follow-up visits. A total of 91 patients who accepted to re-visit for follow-up were retrospectively subjected to the pubertal-stage assessment, developed by J Kaiser et al. [8]. In addition to demographic characteristic of participants, development of facial hairs in boys and the age at menarche in girls was evaluated. Due to the absence of the growth chart of the participants, they were questioned about height conditions during the age range of 12 to 15 years and 15 to 18 years, and also if they experienced conditions of delayed puberty and growth. Data on pubertal development and rate of puberty were obtained. The height (Ht) was measured in a standing position with bare feet using a portable measuring device (SECA 284), and was compared with puberty and pubertal growth of healthy Turkish children.
Statistical Analysis
Data analysis was performed using SPSS version 18.0 for Windows. Descriptive statistical variables were expressed in mean ± standard deviation (mean ± SD) and median range (minimum-maximum). Categorical data were presented as number (n) and precentages (%). The normality of distribution of data was evaluated with the Shapiro-Wilk test and parametric methods were performed to analyze variables which were in normal distribution and equal variance, whereas nonparametric methods were performed to analyze variables which were in normal distribution and unequal variance. The Mann-Whitney U-test and chi-square tests were performed for variables that were not normally distributed while One-way ANOVA was performed for associated samples. “p” values <0.05 were considered statistically significant with a confidence level of 95%.
Results
Of all participants, 10 (12.1%) were females and 81 (87.9%) were males. The mean age was 25.90 ± 6.83 (range, 17 to 38) years and the mean height was 178.90 ± 7.6 (range, 161 to 197) cm. The mean height of the men was 179.8 ± 6.84 (range, 165 to 197) cm, while the mean height of the women was 171.60 ± 9.80 (range, 161 to 196) cm. The mean age at menarche of female participants was 14.20 ± 1.31 (range, 12 to 17) years. The mean age for the development of facial hairs in the men was 16.63 ± 1.69 (range, 13 to 22) years (Table 1).Table 1: Patient demographics.
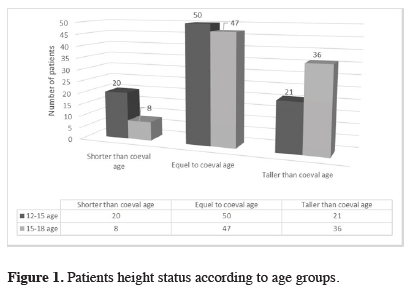
All patients were questioned about their height and puberty conditions during the age range of 12 to 15 years and 15 to 18 years (Table 2). Based on these data, 50 (54.9%) reported to have an average height during the age group of 12 to 15, compared to those of the same age group; 21 (23.1%) were tall and 20 (22.0%) were shorter than those of the same age group. The results showed that individuals between the 15 to 18 age group who described themselves as short decreased from 20 (22.0%) to 8 (8.8%), whereas the number of those who described themselves as tall was found to increase from 21 (23.1%) to 36 (39.6%) (Figure 1). Thirty-nine participants had a sudden increase in height. The peak increase in height of the same age group was found to occur after the age of 16 years. Comparison of the mean age at menarche of women of the study group (14.20 years) with that of Turkish women (12.2 years) [9,10] demonstrated that women of the study group had a statistically delayed onset of menarche (p < 0.0001).
Table 2: Patients height status according to age groups.
 Click Here to Zoom |
Figure 1: Patients height status according to age groups. |
Comparison of the mean age of developing facial hairs in men of the study group (16.63 years) with that of Turkish children (14.4 years) [10,11] demonstrated that men of the study group had a statistically later age for the developing facial hairs (p < 0.0001).
A total of 51 (56%) individuals were reported to smoke, whereas 40 (44%) individuals had no history of cigarette smoking. Of these individuals, 34 were men and six were women. Irrespective of the cigarette smoking status all participants were re-evaluated. A total of 34 men who did not smoke (Group A) had a mean height of 181.32 ± 6.67 (range, 167 to 197) cm and age of developing facial hair of 16.44 ± 1.58 (range, 14 to 21) years. On the other hand, six women who did not smoke (Group B) were reported to have a mean height of 175.50 ± 10.61 (range, 168 to 196) cm and a mean age at menarche of 14.50 ± 1.38 (range, 13 to 17) years. Comparison of participants of Group A and Group B with individuals in the normal population demonstrated that the mean age of developing facial hair and the age at menarche was delayed in the study population (p < 0.0001).
Discussion
After its first description by Itard in 1803, many researches on PSP have been conducted and many hypotheses have been put forth [1,4,12,13]. It has been shown to be a condition which presents itself as a life-threatening crisis and which is mostly observed in young and tall men [1,4]. This condition has been reported to cause discomfort among participants leading to a change in their lifestyle. Although definite factors have been associated with the mechanism of developing PSP, the most important pathological factors are bulla/bleb formations of the lungs [2]. Thoracic computed tomography (CT) is the most commonly used diagnostic tool during investigation of the etiology of spontaneous pneumothorax. Smit et al. [14] identified bulla or bleb formations in 56% of patients with SP and in 64% of patients with recurrent SP; however, they were unable to identify any pathology in 41% of cases. Many studies reported that the size and location of bullae had no effect on the recurrence rates, although some authors found that CT was essential in identifying pathological features for the prediction of recurrence [15]. Although there are hypotheses on the development of these formations, there is no definite prove to better explain why they develop and why they are mostly found in tall men [13].Pneumothorax presents with crises. Literature studies demonstrated that 16 to 52% of recurrent cases developed after the first pneumothorax attack, depending on the type of treatment with recurrence within the first 2 to 24 months and a rate of more than half reported within the first four years [4]. The form of treatment of PSP is, however, still a matter of debate. Pleural drainage during the first PSP attack and follow-up in certain selected cases are usually accepted as the optimal approach [2]. Apart from radiologically small-sized (<20%) and clinically asymptomatic and cases with mild symptoms, pneumothorax is a disease condition which necessitates emergency intervention due to its possible fatal complications. As a result, tube thoracostomy (TT) is the mostly commonly referred treatment method for pneumothorax. However, the guidelines of the British Thoracic Society recommend simple aspiration as the first-line treatment, and suggest that the treatment success rate is similar to TT [16]. On the other hand, in 2001 the American College of Chest Physicians 2001 published that a chest tube or Heimlich valve should be considered as the first-line treatment for PSP patients [17]. In the case of an indication for classical surgical operation for PSP, intrathoracic exploration by videothoracoscopy or thoracotomy would be necessary [G]. In addition, various studies are underway to investigate the method of choice and procedure to prevent recurrence. In a study conducted by Cardillo G et al. [18] to investigate the prevention of the recurrence of pneumothorax, video assisted thoracoscopic surgery (VATS) was implemented with talc poudrage and a recurrence rate of 1.9% was reported. The authors concluded that this rate was significantly higher in cigarette smokers.
In this study, we aimed to move a step further in the etiology of pneumothorax and investigate the causes of this disease. Accordingly, we planned on investigating the growth and development status of mostly young adults. Although participants of our study were about 18-years-old and there was no growth-development chart, we obtained our data using the modified retrospective pubertal-stage assessment form, developed by J Kaiser et al. [8]. In addition to height and weight gain during puberty, changes in the development of secondary sex characteristics, changes in fat and muscle tissue distribution and quantity, and changes in circulation and respiratory system associated with position were reported. This process can be completed with two years in some adolescents, while in others it can take a period of three times more. Menarche is usually the latest physical change observed in girls during puberty. There is a close relationship between menarche and adolescent growth spurt. It is observed after the period, when the growth is fastest. It has been estimated as 12.3 years in the Turkish society [9]. An early age at menarche is known to be associated with short stature during adulthood. The mean age at menarche of 10 women of our study was 14.2 years and this was found to be way more than the known average age at menarche. As observed with menarche in girls, development of hairs in boys was also reported to be around mid-puberty. The adolescent growth spurt curve is observed to peak about two years in women earlier than in men. The short period of adolescent growth spurt forms the peak height velocity (HtV), which is considered as the fastest time of growth. The peak growth rate in girls during adolescence is 8.5 ± 1.5 cm/years, and peak growth age is 11.3 ± 1.5 years [19], in boys the rate is 10.1 ± 1.6 cm/years, while peak growth age is 13.7 ± 0.7 years [20]. In our study, delayed puberty was reported to be related to menarche and facial hair development which are considered as age markers for mid-puberty. Height velocity is often thought to result in menarche and facial hair development, suggesting that delayed HtV is expected in these patients. Furthermore, results from the history of participants of our study showed that increase in height was delayed due to delayed puberty and that there was late growth, compared to their age group. It is possible that this late growth may possible affect tissue and organ development expected to occur during the normal time interval. This negative interaction may be a factor in the development of pneumothorax, particularly considering the fact that lung development lasts until the age of 18 years.
In conclusion, our study results showed that the patients with pneumothorax had an advanced age at puberty, compared to the normal overall population, and that the pubertal growth spurt occurred within a short period of time. This condition is also suggested to have an effect on the lung maturity and the development of pneumothorax.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Reference
1) Fry WA, Paape K. Pneumothorax. In: Shields TW, LoCicero J, Ponn RB, Rusch VW, editors. General thoracic surgery. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2005:794-805.
2) Brown SG, Ball EL, Perrin K, Read CA, Asha SE, Beasley R, et al. Study protocol for a randomised controlled trial of invasive versus conservative management of primary spontaneous pneumothorax. BMJ Open 2016; 13; 6: e011826.
3) Bense L, Eklund G, Wiman LG. Smoking and the increased risk of contracting spontaneous pneumothorax. Chest 1998; 92: 1009-12.
4) Celik B, Nadir A, Sahin E, Kaptanoglu M, Demir H, Furtun K. Riks factors, clinical and radiological evaluation in patients with recurrent spontaneous pneumothorax. Turk Gogus Kalp Dama 2008; 16: 107-12.
5) Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr 1985; 107: 317-29.
6) Butenandt O, Kunze D. Growth Velocity in Constitutional Delay of Growth and Development. J Pediatr Endocrinol Metab 2010; 23: 19-25.
7) Bierich JR. Constitutional delay of growth and adolescence. Baillieres Clin Endocrinol Metab 1992; 6: 573-88.
8) Kaiser J, Gruzelier JH. The Adolescence Scale (AS-ICSM): a tool for the retrospective assessment of puberty milestones. Acta Paediatr Suppl 1999; 88: 64-8.
9) Neyzi O, Alp H, Orhon A. Sexual maturation in Turkish girls. Ann Hum Biol 1975; 2: 49-59.
10) Neyzi O, Furman A, Bundak R, Gunoz H, Darendeliler F, Bas F. Growth references for Turkish children aged 6 to 18 years. Acta Paediatr 2006; 95: 1635-41.
11) Neyzi O, Alp H, Yalcindag A, Yakacikli S, Orphon A. Sexual maturation in Turkish boys. Ann Hum Biol 1975; 2: 251-9.
12) Gupta D, Hansell A, Nichols T, Duong T, Ayres JG, Strachan D. Epidemiology of pneumothorax in England. Thorax 2000; 55: 666-71.
13) Fujino S, Inoue S, Tezuka N, Hanaoka J, Sawai S, Ichinose M, et al. Physical development of surgically treated patients with primary spontaneous pneumothorax. Chest 1999; 116: 899-902.
14) Smit HJ, Wienk MA, Schreurs AJ, Schramel FM, Postmus PE. Do bullae indicate a predisposition to recurrent pneumothorax? Br J Radiol 2000; 73: 356-9.
15) Ouanes-Besbes L, Golli M, Knani J, Dachraoui F, Nciri N, El Atrous S et al. Prediction of recurrent spontaneous pneumothorax: CT scan findings versus management features. Respir Med 2007; 101: 230-6.
16) Henry M, Arnold T, Harvey J; Pleural Diseases Group, Standards of Care Committee, British Thoracic Society. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003; 58 Suppl 2:ii39-52.
17) Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J, et al. Management of spontaneous pneumothorax: an American Collage of Chest Physicians Delphi consensus statement. Chest 2001; 119: 590-602.
18) Cardillo G, Bintcliffe OJ, Carleo F, Carbone L, Di Martino M, Kahan BC, et al. Primary spontaneous pneumothorax: a cohort study of VATS with talc poudrage. Thorax 2016; 71: 847-53.






