

2Department of Thoracic Surgery, Manisa Celal Bayar University, Faculty of Medicine, Manisa, Turkey
3Department of Thoracic Surgery, University of Health Sciences, Dr. Suat Seren Chest Diseases and Chest Surgery Training and Research Hospital, İzmir, Turkey DOI : 10.26663/cts.2020.00017
Summary
Hemothorax occurs due to various conditions such as trauma, malignancy, tuberculosis, bullous lung disease, and lung abscess. In patients with malignant hemothorax, stabilization of the clinical condition and treatment of primary disease is of primary importance. A 53-year-old female patient, who had a history of surgery for ovarian cancer and liver metastasis, admitted to the hospital with complaints of shortness of breath. The patient was found to have pleural effusion on the right hemithorax, and a tube thoracostomy was performed. During the clinical follow-up, the amount of drainage did not decrease. Thereby, the patient underwent a video-thoracoscopic evaluation, and chemical pleurodesis was applied intraoperatively. Despite chemical pleurodesis, hemorrhagic drainage continued. Intrapleural tranexamic acid administration was performed to ensure the stabilization of the clinical condition. Immediately days after the intrapleural application of tranexamic acid, the drainage of the fluid decreased and became sero-hemorrhagic. The management of this case made us think that intrapleural tranexamic acid may be an alternative in persistent malign hemothorax.Introduction
Hemothorax is diagnosed as when the hematocrit level in the pleural fluid is higher than 50% of blood hematocrit. Although the incidence is unknown, the most common cause is trauma [1]. Hemorrhagic effusions also might occur in malignancies, but malignancies rarely cause a massive hemothorax [2]. Malignant effusion was defined as malignant hemothorax with hemorrhagic drainage above 500 cc/day after the pleural malignancy was proven. The management of these patients can sometimes be extremely difficult. Herein, we present a massive malignant hemothorax that was controlled with intrapleural tranexamic acid (TXA).Case Presentation
A 53-year-old female patient with no history of trauma or drug use presented with shortness of breath. She had a history of surgery for ovarian carcinoma eight years ago, and liver metastasis was detected four years after surgery. The chest x-ray revealed right-sided massive pleural effusion (Figure 1).
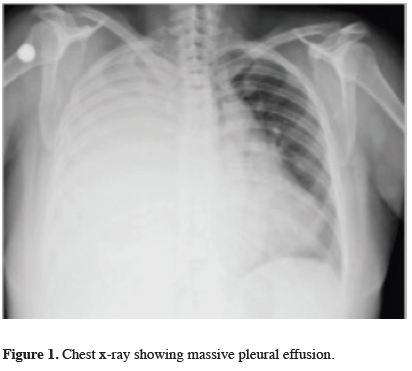 Click Here to Zoom |
Figure 1: Chest x-ray showing massive pleural effusion. |
Thoracentesis revealed a hemothorax, and a tube thoracostomy was performed. Approximately 800-1000 cc fluid per day was drained for five days. Within the follow-up period, the hemoglobin level was dropped to 7 gr/dL, hematocrit to 20%, and liver function tests minimally elevated. Thorax computed tomography demonstrated pleural thickening and loculated effusion (Figure 2).
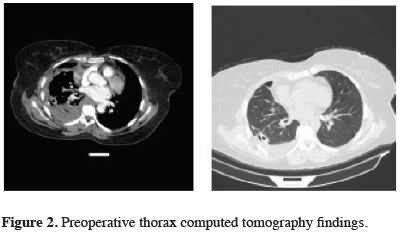 Click Here to Zoom |
Figure 2: Preoperative thorax computed tomography findings. |
The patient underwent a video-thoracoscopic exploration. Hemorrhagic areas spreading the whole pleura were observed. Pleural biopsies were taken, and the frozen section examination revealed pleural malignancy. Pleurodesis was performed with sterile talc insufflation. Definitive histopathologic and cytological examination of pleural fluid and specimens were reported as sarcomatoid carcinoma metastasis. During the postoperative period, since hemorrhagic drainage was continued, two units of packed red blood cells and fresh frozen plasma/day were transfused daily. The hemorrhagic drainage continued approximately 400 cc/day in the first postoperative week. Chest x-rays after the operation and after 2 weeks were demonstrated in Figure 3.
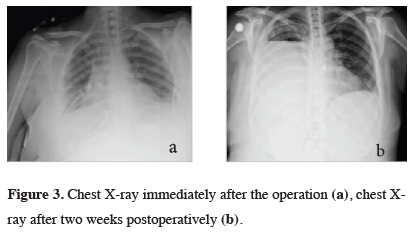 Click Here to Zoom |
Figure 3: Chest X-ray immediately after the operation (a), chest X-ray after two weeks postoperatively (b). |
Since the patient’s general condition was gradually deteriorated, tranexamic acid (Transamine® %10 ampule, Istanbul) 5 g (50 mg/cc) was applied intrapleurally in 250 cc of an isotonic solution, and the chest tube was clamped for 2 hours. Then, the clamping was stopped, and the tube was allowed for free drainage. At the end of the third day, hemorrhagic drainage decreased to 200-300 cc/day and became sero-hemorrhagic. The pleural fluid examination revealed no hemothorax. The patient was referred to the oncology department after the clinical status was stabilized with tube thoracostomy.
Written informed consent was obtained from the patient for publication of her data.
Discussion
In patients with malignant pleural effusions, palliative therapies are preferred due to the lack of complete cure chance with surgical treatment and low life expectancy. In this regard, patients underwent repetitive thoracentesis, intrapleural catheter applications, pleurodesis, and palliative treatments [3]. The British Thoracic Society (BTS) recommends systemic chemotherapy in malignant effusions with asymptomatic and non-specific tumor-related malign effusions [4]. Hemothorax, due to malignancies, is very rare. In our case, the patient had no history of trauma, tuberculosis, or other situations such as lung abscess or bullous lung disease, which could explain the hemorrhagic nature of the pleural effusion. However, she was diagnosed with ovarian carcinoma and had liver metastasis in her history.Schwannoma, thymic lesions, vascular tumors, germ cell tumors, hepatocellular carcinoma (HCC), lung cancer, mesothelioma, primitive neuroectodermal tumors may cause extramedullary hematopoiesis and are involved in the etiology of malignant hemothorax [4]. Tranexamic acid is an antifibrinolytic agent and reduces postoperative bleeding and transfusion risk in cardiothoracic surgery [5]. A synthetic lysine derives of tranexamic acid that binds to lysine binding sites in plasminogen inhibits plasmin activation. Plasmin cannot bind to fibrinogen or other monomers and inhibits fibrinolysis. It also preserves platelet function by inhibiting plasmid-dependent platelet activation. As a result, tranexamic acid prevents the breakdown of the existing clot and does not cause new clot formation.
Other than the hemothorax, the application of tranexamic acid appears in different local application areas in the literature. In patients with chronic inflammatory pleural/pulmonary disease undergoing open surgery intrapleural tranexamic acid administration has been reported to be useful and effective in bleeding control [5].
In the literature, combined oral and intrapleural tranexamic acid was given to two patients with pleural mesothelioma followed by hemothorax. It has been found to reduce the amount of bleeding and the need for transfusion [6]. In our case, both the pleural tissue and fluid were proven to be malignant. The drainage of the patient continued 800-1000 cc per day and the patient was diagnosed as malignant hemothorax.
Intrapleural tranexamic acid application is rarely practiced after pulmonary resections and decortication. However, there are some reports that tranexamic acid may reduce the re-thoracotomy risk when used in these situations [7-8]. The use of intrapleural tranexamic acid in malignant hemothorax is even more uncommon.
In conclusion, in our case, intrapleural tranexamic acid was determined to relieve the malignant hemothorax and improve the patient’s clinical condition since she did not respond to blood and fresh frozen plasma transfusions and the intraoperatively performed talc pleurodesis. Since the patient’s primary treatment was chemotherapy and/or radiotherapy, the main objective should be stabilizing the clinical condition in malignant hemothorax to initiate the routine treatment of the primary disease. In this respect, intrapleural tranexamic acid administration was considered as an alternative method for patients.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Reference
1) Ricketti PA, Unkle DW, Lockey R, Cleri DJ, Ricketti AJ. Case study: idiopathic hemothorax in a patient with status asthmaticus. Journal of Asthma 2016;53:770-73.
2) Morgan CK, Bashoura L, Balachandran D, Faiz SA. Spontaneous hemothorax. Ann Thorac Surg 2015;12:1578-82.
3) Nam HS. Malignant pleural effusion: medical approaches for diagnosis and management. Tuberc Respir Dis 2014;76:211-7.
4) Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:(Supp)32-40.
5) Dunn CJ, Goa KL. Tranexamic acid. Drugs 1999;57:1005-32.
6) De Boer WA, Koolen MG, Roos CM, Ten Cate JW. Tranexamic acid treatment of hemothorax in two patients with malignant mesothelioma. Chest 1991;100:847-8.



