

Summary
Background: We reviewed pre-, intra-, and post-operative clinical data, including morbidity and mortality rates, for patients who underwent Nuss surgery to repair pectus excavatum.Materials and Methods: Nuss procedure was performed in 140 patients at our clinic between 2012 and 2019 (males: 108; females: 32 females; mean age: 15.7 ± 7.8 years; range: 3-40 years). The Haller index was mild (2.5-3.2) in 72, moderate (3.2-3.5) in 40, and severe (3.6-6.0) in 28 patients.
Results: None of the patients died. Morbidity or bad blood loss was not observed. The mean duration of surgery was 59.5 ± 21 min (range: 30-120 min). The mean duration of postoperative hospitalization was 4.6 ± 2.9 days (range: 3-19 days). The procedure was performed using one (n = 107; 76.4%), two (n = 31; 22.2%), or three (n = 2; 1.4%) bars. There were five cases of early pneumothorax, four pleural effusions, five bar displacements, two wound infections, one hematoma, seven bar exposures due to allergies, and three costal fractures.
Conclusions: The Nuss operation is an effective, minimally-invasive treatment for pectus excavatum. It has the advantages of a short duration of surgery and low morbidity and mortality rates. Therefore, the Nuss operation may be considered the treatment of choice in cases of pectus excavatum.
Introduction
Pectus excavatum (PE) is the most common congenital chest wall disorder, with an overall incidence of 0.1%. The male to female ratio for PE is 4:1 [1]. The traditional repair technique is open sternoplasty [2]. PE progresses during puberty [3]. In 1998, Donald Nuss first used minimally invasive surgery to repair 45 cases of PE [1]. In this procedure, a metal bar is inserted into the retrosternal area using a thoracoscope and later removed [2]. Connective tissue diseases, including Marfan and Ehler-Danlos syndrome, accompany PE in 6% of patients, while scoliosis is seen in 40-65% of cases. Patients may show diminished exercise capacity, fatigue, dyspnea on deep inspiration, and recurrent pulmonary infections. Surgery is important because the negative aesthetics reduce self-confidence, which can cause social problems [4]. Preoperatively, the transverse thorax diameter is divided by the sternovertebral distance on thoracic tomography to obtain the Haller index (HI). An HI > 3.25 indicates the need for surgery. Kelly considered the following as indications for surgery: HI > 3.25, major displacement of the heart and/or cardiac compression, pulmonary compression, restrictive ventilation, mitral valve prolapse, bundle branch block or another cardiac pathology related to cardiac compression, or a history of ineffective correction [5]. However, most patients are operated upon due to aesthetic concerns.We analyzed the data of patients who underwent the Nuss procedure in our clinic between 2012 and 2019.
Methods
Written informed consent was obtained from all adult patients and the parents of young patients for the study. We analyzed a retrospective cohort study. Our institutional ethics committee approved the study (Approval no: 2020/01). We enrolled 140 patients (108 males and 32 females with a mean age of 15.7 ± 7.8 years (range: 3-40 years); one patient was 2.5-5 years of age, 28 were aged 6-12 years, 82 were aged 13-18 years, 19 were aged 19-25 years, and 11 were aged 25-40 years. One patient had previously undergone Abramson surgery; two others had undergone wedge resection to treat pulmonary sequestration and bronchiectasis, and one had undergone bullectomy. We obtained the patients’ medical histories and performed electrocardiography, echography, direct graphing, thoracic computed tomography, and pulmonary function tests prior to surgery. Preoperative clinical symptoms, including dyspnea on effort, chest pain, fatigue, and palpitations, were evident in 79 patients (56.4%); 108 (77.1%) patients had pulmonary function disorders, 31 (22.1%) had mildly restrictive ventilation dysfunction, 51 (36.4%) had moderately restrictive ventilation dysfunction, 6 (18.5%) had severely restrictive ventilation dysfunction, 27 (19.2%) had echocardiographic abnormalities, 10 (7.1%) had mitral valve prolapse, 4 (2.8%) had mild mitral insufficiency, 2 (1.4%) had patent foramen ovale (Table 1).Table 1: Demographic and clinical data of the pectus excavatum patients before surgery.
Patients were classified according to their condition using the HI, as mild (HI = 2.5-3.2; n = 72, 51.4%), moderate (HI = 3.2-3.5; n = 51, 36.4%),or severe (HI = 3.6-6.0; n = 28, 20%). Comorbidities were present in 18 (12.8%) patients, including eight with pterygoid chests, 12 with scoliosis, one with pulmonary sequestration, one with asthma, one with coarctation of aorta, two with Marfan syndrome, and one with growth retardation (Table 2).
Table 2: Clinical data of the pectus excavatum pa-tients before surgery.
Surgical indications
The criteria for surgery were as follows: pectus index (PI) > 3.25, requirement for cosmetic defect correction, major displacement of the heart and/or cardiac compression, pulmonary compression, a restrictive ventilation pattern, mitral valve prolapse, a bundle branch block or other cardiac pathology related to cardiac compression, and a history of unsuccessful operations.
Surgical procedure
All patients were placed under general anesthesia via single-lumen endotracheal intubation. The preoperatively measured Nuss steel bars fitted the chest wall well. Bars were made of surgical steel and were 13 mm wide and 2 mm thick (Hipokrat®, Izmir, Turkey).Three-centimeter-long transverse incisions were created on both sides of the thorax, running from the beginning to the anterior axillary line to where it met the deepest point of the depression. After dissection of subcutaneous tissue and muscle, the tissue along the rib surface up to the highest hinge point was manually dissected. A camera port was inserted in the midaxillary line of the seventh intercostal area. Under videothoracoscopic guidance and carbon dioxide insufflation, an introducer was placed from the highest hinge point to the right thoracic cavity, and then passed from the right mediastinum to the left chest wall and then to the highest contralateral hinge point. The introducer was then grasped and pressed against the sternum to shape the chest contour. Nylon tape was adhered to the end of the introducer. After the introducer had been extracted from the left thoracic wall, the Nuss bar was bound to the end of the tape and the bar was pulled back to the contralateral side of the chest wall using a rope. The bar was then inverted to lift the sternum. Two fixation stabilizers were placed on the tips of the bar. A feeding tube was placed in the right chest wall and the lung was inflated to remove the artificial pneumothorax. In patients with extremely severe PE, a small incision was created under the xiphoid to allow the surgeon’s fingers to guide the introducer. Alternatively, a crane was used to elevate the sternum.
Postoperative treatment
Epidural analgesia was used to relieve pain, which is the most serious postoperative problem. If an epidural catheter could not be inserted, intravenous analgesia was used. Intravenous antibiotics were given early on the first postoperative day. Patients were rapidly mobilized and discharged after 3–7 days in hospital. They were instructed not to lift heavy objects or perform vigorous exercise for 2 months. They were followed-up regularly and the bars were extracted after 3 years.
Results
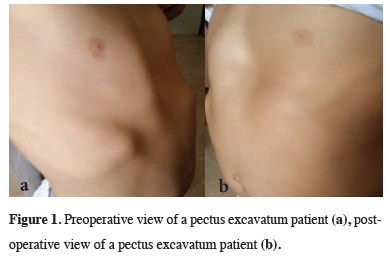
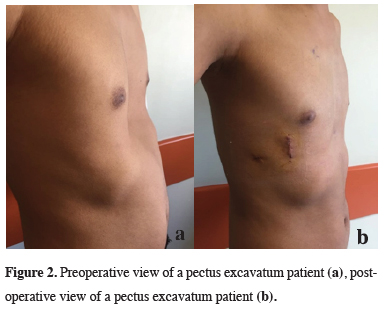
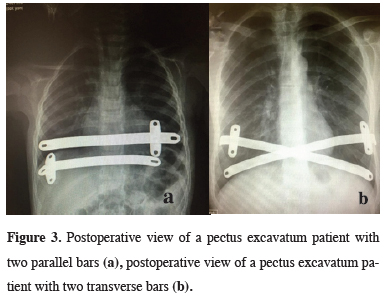
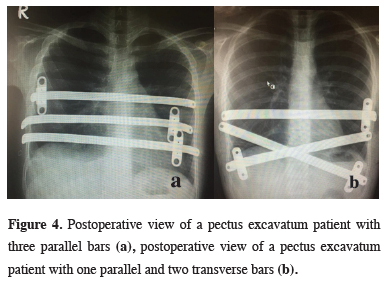
In all 140 patients, surgery was successful, and morbidity or blood loss was not observed (Figures 1,2). On computed tomography scans, the mean HI was 4.5 ± 1.4 (range: 2.8-6.2). The mean duration of surgery was 39.5 ± 21 min (range: 30-120 min). The postoperative mean duration of hospitalization was 4.6 ± 2.9 days (range: 3-19 days). No pericardial perforation was noted. Three patients underwent Abramson surgery using the sandwich technique, while one underwent thoracoscopic bullectomy and another pulmonary wedge resection. A total of 107(76.4%) patients received one bar, while 31(22.2%) received two bars, and 2(1.4%) received three bars. No patient received more than three bars. Two or three bars were placed for larger affected areas on the 93 chest wall (Figures 3,4).
 Click Here to Zoom |
Figure 1: Preoperative view of a pectus excavatum patient (a), postoperative view of a pectus excavatum patient (b). |
 Click Here to Zoom |
Figure 2: Preoperative view of a pectus excavatum patient (a), postoperative view of a pectus excavatum patient (b). |
 Click Here to Zoom |
Figure 3: Postoperative view of a pectus excavatum patient with two parallel bars (a), postoperative view of a pectus excavatum patient with two transverse bars (b). |
 Click Here to Zoom |
Figure 4: Postoperative view of a pectus excavatum patient with three parallel bars (a), postoperative view of a pectus excavatum patient with one parallel and two transverse bars (b). |
In terms of postoperative complications, early pneumothorax was seen in five patients, of whom two underwent tube thoracostomy. Pleural effusion was evident in four patients, but treatment was not necessary. Postoperative bar displacement was evident in five patients, so the bars were removed 20-23 months early. The bars were also removed early in patients who complained of painful scoliosis. Wound infections were noted in two patients, both of whom responded to antibiotics. A subcutaneous hematoma was observed in one patient, and debridement was performed. Bar exposure caused by allergy was noted in seven patients, and costal fractures were seen in three others (Table 3). Allergy developed to Nuss steel 101 bars with no. 14 and 15. Tip of the bars got out of the skin in the patients with bar allergy. Surgical 102 wound infection was seen in 2 patients. Bars were removed in 3 patients due to allergy, 4 patients with bar allergy were treated with surgical wound revision and antibiotics. Vacuum-assisted wound closure was applied in one patient. Patients were followed up at 1, 3, 6, and 12 months. The mean follow-up time was 43 ± 26.3 months (range: 1-93 months). There were no cases of obvious recurrence.
Discussion
PE is a progressive genetic deformity of the cartilaginous regions of the ribs and sternum, characterized by some degree of depression of the anterior chest wall [2-5]. The male:female ratio of PE was reported at 4:1[3]; in our study, it was 3.3:1. Although females are less frequently affected, their deformities tend to be more severe. Marfan syndrome was evident in 15% of patients [3]. As the risk of PE recurrence is much higher in such patients, Marfan syndrome status requires careful evaluation [4]. Two of our patients had Marfan syndrome, but no postoperative recurrence was noted. Among all patients, 26% had scoliosis and 12(8.4%) had scoliosislike deformities. The patients were generally asymptomatic, although some complained of chest pain, fatigue, dyspnea, palpitations, and movement limitations. Seventy-nine patients (56.4%) were symptomatic. Congenital cardiac defects, especially mitral valve prolapse, may accompany PE [2]. Twenty-seven patients showed preoperative echocardiographic abnormalities and ten (7.1%) had mitral valve prolapse.Ravitch sternoplasty is the traditional method for surgical correction of PE [6]. In 1949, Ravitch was the first to excise all deformed cartilage and perichondrium, decompose the xyphoid and intercostal packages from the sternum, and perform right sternal alignment accompanied by transverse sternal osteotomy. Kirschner wires were used for fixation; silk sutures were later preferred [2]. However, these techniques caused pain, and were associated with long operative and hospitalization times, bleeding, many postoperative complications, and poor cosmetic outcomes.
In 1987, Donald Nuss, a pediatric surgeon, described a minimally invasive method that did not involve osteotomy. The ideal patient age for the procedure was considered to be 6-12 years, where PE involves the costal cartilages [1]. Also, the psychological impact of PE is less in children. Our patients were aged between 3-40 years (mean age, 15.7 ± 7.8 years). Only one patient was aged under 6 years; that 3-year-old patient was operated upon because a severe deformity depressed the heart. Although the technique is more difficult to apply in older patients with stronger chest walls, selected patients may benefit.
The Nuss technique is associated with good cosmetic results, short surgery, no blood loss, and fast recovery. The mean duration of surgery in our study was 39.5 ± 21 min, which is markedly shorter than that for the traditional technique. Cardiac injury is the most important potential complication. We observed no postoperative bleeding or pericardial perforation in our patients, none of whom died. The thoracoscopic view was excellent in all patients and we were able to protect the heart. Carbondioxide insufflation allowed us to proceed beyond the adhesive bands that lie behind the sternum; this further reduced the risk of cardiac damage. For severe PE cases, a small incision created below the xyphoid or sternum allows the surgeon to elevate, and deal with adhesions behind, the sternum.
The Nuss technique is valuable for patients who develop recurrence after open sternoplasty. However, these patients require careful evaluation because retrosternal adhesions may be present [2]. None of our cases had a history of open surgery.
The most common complications of Nuss surgery are pleural effusions, bar displacement, wound infection, pneumothorax, scoliosis, and bar allergy. Zhang et al reported a postoperative complication rate of 9.7% in 639 patients [1]. The rate in our study was similar, at 10.7%. Nuss reported that the most common complication among their 1,015 cases was pneumothorax (60.4%) that spontaneously resolved [7]. The most common complication among our patients was bar exposure caused by bar allergy (5%). Pneumothorax (3.5% of patients) was the second most common complication; however, this was diagnosed by chest X-ray only. Nuss reported that 3.6% of pneumothorax cases required chest drainage, compared to a rate of 1.4% in our study.
In a Canadian study, postoperative pleural effusion developed in 4.65% of patients; in our study, the rate was 2.8%, while the wound infection rate was 1.4%.Postoperative pneumothorax developed in 3% of patients in their study; in our study, 151 the rate was %3.5. Bar displacement was seen in %3.7 in their study; in our study, the rate was 152 %3.5. In patients with relatively large affected areas, we placed two or three bars (fixed with 153 stabilizers) on the right and left tips; the stabilizers usually prevent bar dislocation. In patients with relatively large affected areas, we placed two or three bars (fixed with stabilizers) on the right and left tips; the stabilizers usually prevent bar dislocation. Postoperative bar displacement was evident in five patients. We did not routinely place chest tubes after surgery; instead, we inflated the lungs and extracted all air from feeding tubes that were temporarily placed in the right hemithorax during surgery.
All steel bars were removed after 3 years. Two patients could not tolerate the bars due to pain. Seven patients were allergic to the bar material and thus exhibited delayed wound-healing. Postoperative bar displacement was evident in five patients, so the bars were removed 20-23 months early. The bars were also removed early in patients who complained of painful scoliosis. Overall, the results were satisfactory.
The Nuss technique requires only small incisions; moreover, the operation is quick, there is no bleeding, and the skeleton can develop in the absence of cartilage. Also, the complication rate is low and the outcome is usually good.
In conclusion, the Nuss technique is an advanced surgical method, although the postoperative outcomes in cases of bar removal require further study, as do the effects of the procedure on cardiopulmonary function [8-10]. A limitation of our study was its single-center, retrospective design. The surgical technique used should be determined on an individual patient basis, i.e., according to general condition and age; more research is still required.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Ethics Approval
The study was approved by the institutional ethical board of Health Sciences University, Istanbul Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Training and Research Hospital (No:01/2020).
Authors’ contributions
TA, MA; co-wrote the paper, collected the data, performed the analysis, contributed data/analysis tools, conceived and designed the analysis.
Reference
1) Zhang D, Tang J, Ben X, Xie L, Zhou H, Ye X et al. Surgical correction of 639 pectus excavatum cases via the Nuss procedure. J Thorac Dis 2015; 7: 1595-605.
2) Yüksel M, Bostancı K, Özalper H. Pektus ekskavatum deformitesinde minimal invazif cerrahi (Nuss Yöntemi). JCAM 2009; 1: 70-4.
3) Nuss D. Pectus excavatum from a pediatric surgeon’s perspective. Ann Cardiothorac Surg 2016; 5: 493-500.
4) Bilgin M. Pectus excavatum: Nuss experience in 110 cases. Turk J Thorac Cardiovasc Surg 2014; 22: 790-4.
5) toraks.org[Internet]. Minimal invaziv pektus ekskavatum düzeltme ameliyatı: Teknik ve uygulama, Marmara Deneyimi; c2011 [cited 2011]. Available from: https://www.toraks.org/.
6) Dübüş T. Pektus ekskavatum tamirinde Nuss tekniği. Ist Med J 2011; 12: 89-95.
7) Nuss D. Minimally invasive surgical repair of pectus excavatum. Semin Pediatr Surg 2008; 17: 209-17.
8) Maagaard M, Tang M, Ringgaard S, Nielsen HH, Frokier J, Haubuf M et al. Normalized cardiopulmonary exercise function in patients with pectus excavatum three years after operation. Ann Thorac Surg 2013; 96: 272-8.



