

Summary
Background: Explantation of totally implantable venous access port (TIVAP) is of great importance as it may interrupt treatment in oncology patients. The aim of this study is to determine the reasons of TIVAP explantation, the relationship between TIVAP in situ time and early explantation for these reasons, and also to analyze the effect of increasing experience over time on these results.Materials and Methods: Patients who underwent TIVAP explantation between 2014 and 2020 were retrospectively reviewed. Demographic characteristics of the patients, indications for TIVAP implantation and explantation, the TIVAPs’ in situ time, early explantation rate and post-explant complications were investigated. The results obtained were compared in two time periods (January 2014-June 2017 and July 2017-December 2020).
Results: A total of 90 patients were analyzed. The mean age was 58.7 ± 10.2. While TIVAP implantation was most frequently performed for digestive tract cancers (73%), the most common cause of TIVAP explantation was an infection (53.3%). In patients with TIVAP explant due to infection, mean TIVAP in situ time (73 days) was significantly shorter compared to other reasons (p < 0.001). In contrast, the early explantation rate due to infection was only 16.6%. Hematoma was the most common post-explant complications, with a total complication rate of 13%. In the time, it was determined that explantations secondary to complications, early explantations and post-explant complications decreased, while TIVAP in situ time increased.
Conclusions: Infection is the reason of more than half of TIVAP explantations. Although infection significantly decreases the TIVAP survival, it rarely causes early explantation. It is important that TIVAP-related processes are performed in multidisciplinary centers and with experienced staff.
Introduction
Total implantable venous access port (TIVAP; also called port in brief) plays a major role in improving the quality of life and safety of patients, as that allow long-term intravenous access for chemotherapy, antibiotherapy, blood transfusion and nutritional products, and offers the advantage of obtaining venous blood samples without having to repeatedly puncture the vein [1,2]. Because the port is placed subcutaneously, it doesn’t restrict daily activities by affecting the range of motion and is less prone to infections than non-totally implantable catheter [3]. Due to these characteristics, TIVAP has become an ideal tool for long-term treatments, especially cancer treatment, and is being used with increasing frequency [4].Despite its benefits, TIVAP is also not entirely free from complications. TIVAP, which may be associated with early (hemothorax, pneumothorax, injury to major blood vessels, air embolism, cardiac arrhythmia, catheter misplacement, etc.) and late (infections, venous thrombosis, extravasation of cytotoxic drugs, mechanical dysfunction of the catheter, port-inversion, skin necrosis, etc.) complications, may need to be explanted for various reasons [5,6]. Otherwise, complicated TIVAP left in situ may cause more serious clinical consequences [7]. Apart from complications, patients may also want TIVAP removed or it can be explanted because this is no longer needed [5,6]. However, this TIVAP explantation is of great importance as it may cause delay or interruption of treatment in patients who continue to need chemotherapy. It is also known that the complications that cause TIVAP explantation are closely related to the increased labor and costs of the health system [3].
The aim of this study is to determine the reasons of TIVAP explantation, the relationship between TIVAP in situ time and early explantation for these reasons, and also to analyze the effect of increasing experience over time on these results in oncology patients.
Methods
Study designAfter obtaining approval from the Local Ethics Committee for Non-Interventional Clinical Studies (protocol number: 2022-49-7164-E), a retrospective cohort study was conducted at a level II private healthcare facility. Informed consent was obtained from all patients.
Study population
For the study population, patients over the age of 18 who underwent TIVAP explantation by a single surgeon in the thoracic surgery department between January 2014 and December 2020 were examined. The selection flow diagram for the study population according to the eligibility criteria is shown in figure 1.
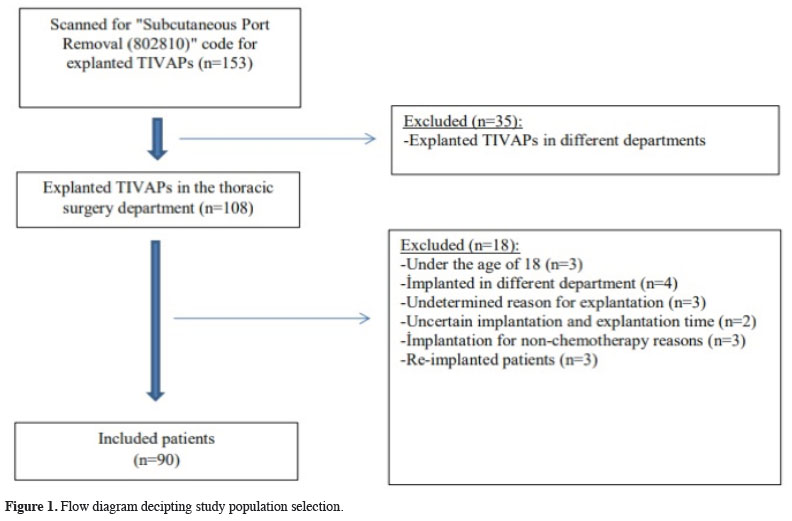 Click Here to Zoom |
Figure 1: Flow diagram decipting study population selection. |
Variables, outcomes
For each patient included in the study, data at explantation such as age, gender, BMI (Body Mass Index), indication for TIVAP explantation, microbiological data, the TIVAP in situ time, CRP (C-reactive protein) level, WBC (white blood cell) count, early explantation rate were obtained. In addition, diabetes mellitus, hypoalbuminemia, leukopenia and thrombocytopenia during implantation, TIVAP implantation indication, TIVAP implantation status, implanted TIVAP brands and post-explant complications were recorded. Primary outcomes were the reasons and frequencies of explantation of TIVAP. Secondary outcomes were the effects of explantation reasons on the TIVAP in situ time, infection parameters during explantation, reason-based early TIVAP explantation rates, and the change in outcomes in two time periods (January 2014 - June 2017 and July 2017 - December 2020).
Data collection
Data of patients who underwent TIVAP explantation were obtained from patient files and the online hospital documentation system. For this purpose, procedure reports codenamed “subcutaneous port removal”, anamnesis notes of the relevant patient, epicrisis reports, constantly updated nurse clinical observation notes or consultation records were reviewed in detail.
Definitions
The reason for explantation was determined as infection in cases with fever in the clinical course before the removal procedure, with signs of stiffness, redness, tenderness, and pain on the implantation site or in cases where pathogenic microorganisms grew in one of the cultures (blood, catheter or exudate culture) taken in order to detect the fever focus (without an obvious source in another region) [7]. At the same time, the frequencies of isolated pathogens were determined in the culture studies of these cases. Dysfunctional ports were evaluated as thrombosis if they had a clinical (edema at upper extremity, swelling or pain at neck) and diagnostic (doppler sonography or thorax computerized tomography scan with contrast enhancement) findings [7]. Dysfunction was defined as dislocation, disconnection/breakage of TIVAP elements, displacement of the catheter tip towards the contralateral subclavian vein or internal jugular vein, extravasation of administered drugs, despite washing with heparinized saline solution, inability to aspirate or infuse fluids. In patients whose chemotherapy was ended, the reason for explantation was recorded as end of treatment. TIVAP explantation secondary to perforation of the skin over the reservoir without signs of infection was defined as skin dehiscence, and TIVAP explantation due to subjective ailments such as discomfort, pain, difficulty in moving the neck or shoulder, and aesthetic problems was defined as patient request. The TIVAP in situ time was defined as the number of catheter days from implantation to explantation [1], and explantation of the TIVAP within 30 days after implantation was defined as early explantation [8]. For biochemical parameters, WBC <3.500 cells/μL was defined as leukopenia, platelet count <150.000/μL was defined as thrombocytopenia, serum albumin level <3.5 mg/dL was defined as hypoalbuminemia [9]. For implantation status, patients who were hospitalized in any service during port placement were designated as inpatients, while the patients who admitted to the thoracic surgery outpatient clinic for port placement, were discharged on the same day after the procedure and did not receive any service admissions were designated as outpatients [10].
Perioperative management
In our hospital, TIVAPs are applied when long-term intravenous transportation is required for chemotherapy and blood transfusions. After oncology and hematology patients are referred from the relevant department for TIVAP implantation according to chemotherapy protocols, implantation is performed by a thoracic surgeon or cardiovascular surgeon. Attention is paid to the fact that the explantation process is also carried out by the same surgeon that performs the implantation process.
All TIVAP implantations were performed in the operating room by creating a subcutaneous pocket on the pectoral muscle through a 3 cm skin incision, under local anesthesia (10-20 mL bupivacaine 0.5%) and moderate sedation with titrated midazolam. Neither prophylactic antibiotics nor routine anti-coagulation therapy was administered [1]. Single-lumen port catheters with a diameter of 8 or 8.5 fr (Secureport®, Plan 1 Health Srl. Amaro, Udine, Italy; PowerPort®, Bard Access Systems, Inc. Salt Lake City, Utah, USA; Celsite®, Braun Medical, Boulogne Cedex, France) were applied to all patients. In most cases, the right subclavian vein was preferred with the seldinger technique as the catheterization site. However, in the presence of mastectomy or radiation scarring, the left subclavian vein, and in case of technical failure, ultrasound-guided internal jugular vein was used for implantation [13]. After implantation, the catheter was flushed with normal saline, the reservoir was filled with diluted heparin, and no antibiotic locks were used. After the procedure, radiographic imaging was performed under fluoroscopy to check the position of the catheter tip in the superior vena cava.
Patients were re-evaluated one week after TIVAP implantation for control of the operative site. After each infusion and every four weeks after chemotherapy treatment is finished, the port was flushed with heparinized normal saline (500 U heparin in 10 cc normal saline). Care and dressings of TIVAPs were managed according to guidelines for the prevention of intravascular catheter-associated infections [11]. Wound infection of the patients was evaluated according to the Surgical Site Infection Prevention Guidelines criteria [12]. The port area was evaluated for infection during each dressing, and wound swabs and blood steam cultures were taken for definitive diagnosis in suspicious cases. While superficial/local wound infections are treated with appropriate antimicrobials, debridement and abscess drainage, in case of port or catheter dehiscence due to wound infection or systemic infection secondary to catheter infection, explantation of TIVAPs were considered [2]. Patients whose catheters were occluded due to thrombosis and who did not respond to the anti-coagulant treatment were also considered for TIVAP explantation.
TIVAP explantation
The explantation procedure was performed under local anesthesia, from the previous incision, with sterile conditions. During TIVAP explantation, the pseudo-capsule consisting of fibrous tissue around the port and the catheter was released with blunt and sharp dissections and then opened with a scalpel. Adhesions in the radio-opaque catheter lock area connecting the port and the catheter were cut with a scalpel to ensure full mobilization of the device, and the port and catheter were removed together. After TIVAP was completely explanted, the remaining pieces of fibrous connective tissue in the subcutaneous area were removed. Then, the subcutaneous tissues were sutured with absorbable 3/0 polyglactin (Vicryl; Ethicon, Somerville, New Jersey) and the skin tissue was sutured with 2/0 polypropylene (Prolene; Ethicon, Somerville, New Jersey). Finally, the sterile dressing was applied with a pressure bandage on the formed pocket. Antibiotic prophylaxis was not given for the explantation procedure either.
Statistical Analysis
Descriptive statistics were expressed as frequency, percentage. Categorical variables were analyzed by Pearson chi-square and Fischer exact or Pearson Chi-Square tests. The normality of numeric variables was tested with the Shapiro Wilk test. Mean differences between two groups with normally distributed will be compared by Student’s t-test, whereas the Mann–Whitney U test will be applied for comparisons of the abnormally distributed data. Kruskal-Wallis test (post hoc test: Bonferroni corrected Mann Whitney U test) is a non-parametric statistical test that evaluates whether two or more samples are drawn from the same distribution. All statistical analyses were performed by using SPSS (Statistical Package for the Social Sciences, SPSS Inc., Chicago, IL, USA) 21.0 package program. P < 0.05 were considered as statistically significant.
Results
Demographic and clinical characteristics of patientsA total of 90 patients undergoing TIVAP explantation were analyzed for the study. The mean age was 58.7 ± 10.2 years (range, 32-77 years), 51% of the patients were male, 49% were female, and the median BMI was 28.7 kg/m2. Most patients did not have diabetes mellitus (86.7%), hypoalbuminemia (95.6%), leukopenia (96.7%), thrombocytopenia (91.1%) at the time of implantation. It was determined that implantation was performed due to the main digestive tract cancers (73%) [colo-rectal cancer (60%) and gastro-esophageal cancer (13%)]. The majority of patients (73.3%) had TIVAPs placed as outpatients. The distribution of explanted TIVAP brands differed (Table 1).
Reasons for TIVAP explantation & isolated microorganisms
The most common reason of TIVAP explantation (53.3%) was an infection. This was followed by the end of the treatment program (17.8%) (Table 2). Wound cultures, blood cultures, or catheter tip cultures were obtained from all patients with TIVAP-associated infections for further microbiological testing. Evidence of bacterial or fungal growth was found in 36 of 48 patients (75%) with infection due to TIVAP explantation. The most common (16.7%) isolated pathogen in cultures was Staphylococcus Aureus. It was determined that the most common (37.5%) isolated pathogens as a group were members of the Gram-positive cocci family. It was determined that these were followed by Gram-negative bacilli (20.8%). Identified pathogens are shown in table 3. In addition, it was determined that the catheter tip and blood culture results were positive with the same microorganisms in 9 cases.
Table 2: Reasons for TIVAP explantation.
Table 3: Microorganisms isolated from patients un-dergoing TIVAP explantation due to infection.
TIVAP in situ time, infection parameters and early explantation
The mean TIVAP in situ time was 215.2 ± 330 days (range, 5-1351 days) in all patients included in the study. It was found that the mean TIVAP in situ time was 73 days in patients who underwent TIVAP explantation due to infection, which was significantly shorter compared to patients who underwent TIVAP explantation for other reasons (p < 0.001, Kruskal-Wallis test) (Table 4). In addition, these patients had significantly higher CRP levels during explantation compared to other reasons (p = 0.002, Kruskal-Wallis test) (Table 4). Although only 16.6% of patients who underwent TIVAP explantation due to infection had the port removed in the early period, the majority of patients (66%) who underwent TIVAP explantation due to technical reasons causing dysfunction [displacement of the catheter towards the contralateral subclavian vein or internal jugular vein (n = 2, 2.2%), hematoma (n = 2, 2.2%), kinking of the catheter (n = 1, 1.1%), extravasation (n = 1, 1.1%)] were performed in the early period (p = 0.003, Exact Chi Square test) (Table 4).
Post-explant complications
Peroperative complications were observed in 12 (13.3%) of 90 patients who underwent TIVAP explantation. Among these, hemorrhage and hematoma occurred at the incision site in 6 patients, transection of the catheter in 3 patients, and wound dehiscence in 3 patients. Surgical revision was required in five patients (four patients with bleeding/hematoma, one patient with wound dehiscence). Three transected catheters were removed by interventional radiology. In 10 patients, venous access incision opened, further dissection and traction were required to mobilize the catheter since the catheter could not be easily removed. However, none of the patients required venotomy to mobilize the catheter.
Comparison of results in two time periods
When the results of the study were analyzed in two time periods (49 explantation procedures were performed in the first period and 41 in the second period), it was determined that the explantations secondary to complications decreased, and the explantations due to the end of the treatment and patient request increased in the next 3-year period. In addition, in the second period, it was determined that TIVAP in situ time increased and early explantations and post-explant complications decreased (Table 5)
Table 5: Comparison of TIVAP explantation reasons and TIVAP in situ time over two time periods.
Discussion
In this study, in which a reason-based analysis of oncology patients who underwent TIVAP explantation was performed, attention is drawn to 3 main findings. The first one is that infection is the most common cause of TIVAP explantation. The second one is that the factor that shortens TIVAP in situ time the most among explantation causes is infection. On the other hand, the rate of early TIVAP explantation due to infection is low. The third and the last one is that TIVAP in situ time increases with increasing experience over time, while the frequency of early TIVAP explantation decreases.As with all medical devices, the ideal expectation from TIVAP in cancer patients is that it can be processed smoothly from the moment its implanted to the end of the treatment [8]. However, TIVAP may be associated with various complications during its stay in the body. A number of studies have shown that TIVAPs are complicated in a wide range of 1.6-28% [13]. Complications such as infection and vein thrombosis usually render TIVAP unusable and removal of TIVAP becomes inevitable. Otherwise, some undesirable consequences may be encountered, such as progression of the infection to severe sepsis or septic shock, or dissemination of vein thrombosis leading to superior vena cava syndrome [14]. However, TIVAPs do not have to be removed only when they are complicated. Explantation can also be performed for some patient-related reasons such as the end of the chemotherapy or (although not recommended ) aesthetic concern [14]. In conclusion, the infection has been shown to be the main reason for TIVAPs explantation in many studies [7,14]. In the series of Biacchi et al [15], the percentage of patients whose TIVAPs were explanted because the therapeutic program ended was only 12%, while the remaining 88% consisted of patients whose TIVAPs were explanted due to various side effects and complications. In this series, infection was reported as the most common reason of TIVAP explantation with a rate of 47%. In another series, the percentage of patients whose ports were removed because the treatment program was ended was 15%, while the most common cause of port removal was infection with a rate of 53% [14]. In our study, the most common reason of explantation was infection with a rate of 53%, while explantation due to end of treatment was in the second place with a rate of 17%, and the results are consistent with the literature. In this context, as a subjective impression, it can be said that these infections may be related to the inappropriate daily use of TIVAP (i.e., no flushing of the device, especially after administering artificial nutrition).
TIVAP-associated infections have been reported to be associated with internal lumen contamination following repeated puncture of the port chamber from the outside during blood collection, washing, or delivery of biological materials [6]. For these reasons, coagulase-negative staphylococci and S. aureus, which are among the natural members of the skin flora, are the most frequently isolated pathogens in port-acquired infections [6]. In addition, recently, gram-negative bacilli, Pseudomonas aeruginosa, and fungi have also begun to be isolated with increasing frequency [6]. In this study, S. aureus and coagulase-negative staphylococci were the most frequently isolated pathogens in patients who underwent TIVAP explantation due to infection, consistent with the results of the literature. Gram-negative bacilli and fungi followed them as a group.
TIVAPs are known to be medical devices with a long lifespan of up to 12 years [15]. In the case of TIVAP-associated infection, our approach is to remove it if there is no response to medical treatment and, if necessary, to insert a new port from the opposite side. In this study, it was determined that the TIVAP in situ time in patients who underwent TIVAP explantation due to infection was significantly shorter compared to patients who underwent TIVAP explantation for other reasons. This may be due to TIVAP explantation as soon as possible after detection of TIVAP-associated infection. Otherwise, it is known that TIVAP-associated infection can result in high mortality and morbidity [14]. On the other hand, it may also mean that, TIVAP explantation is not performed quickly (as in infection) in case of non-infectious complications. In support of this practice, it is recommended to try various ‘rescue therapy’ methods first for such complications, and to remove TIVAPs only when they are not necessary or non-functional [16]. As a result, it is understandable that there is such a difference between the duration of the port stay in the body in terms of reasons of TIVAP explantation.
In the study, it was determined that the early TIVAP explantation rate was low (15%). The most important reason for this may be that TIVAP explantation rate due to infection (the most common reason) is very low (16.6%) in the early period. Various studies have identified some factors that increase the risk of TIVAP-related infections in the early period. These factors are listed as presence of hematological malignancy, hypoalbuminemia, leukopenia, thrombocytopenia, high INR, diabetes mellitus, inpatient port placement, and port placement in the neonatal and infant period [9,17-20]. The fact that the listed risk factors were absent or very few in our patient group indicates that the risk of TIVAP-related infections in these patients is low in the early period. In addition, it has been shown that up to 50% of early period TIVAP-associated infections can be prevented with the implementation of various multimodal programs that include the education of healthcare providers and patients [21]. Similar programs, which are increasingly being implemented in our hospital, may also have contributed to the low rate of TIVAP explantation due to an infection in the early period.
In general, TIVAP explantations are considered minor surgical interventions. In a limited number of studies in the literature, it has been reported that the rate of peroperative complications associated with this procedure ranged from 2.6% to 16% [7,22]. In addition, it has been reported that TIVAPs may not be easily removed due to infection, fibrous sheath reaction, fixation to the vessel wall, dense adherent calcifications, post-thrombotic adhesions, long catheter length and a long stay in the body, and even if the catheter fragment is retained, this can have serious consequences, including death, following embolization to the heart or pulmonary arteries [7,22,23]. Therefore, it has been mentioned that interventional radiology may be needed at any time during the procedure, and even open surgery (venotomy, sternotomy, etc.) may be required in cases with high explantation difficulty [22]. In this study, the peroperative complication rate associated with the explantation of TIVAP was 13%, which is comparable to the reported complication rates. The main complication for these cases was post-procedure bleeding/hematoma. In addition, interventional radiology was required for three transected catheters during the procedure, and advanced surgical dissection was required for 10 patients whose catheters could not be removed despite severe traction. In order to reduce these peroperative complications, it is recommended not to use polyurethane catheters, especially for protocols that require long-term implantation [24], and to use electric blades that cannot penetrate the catheter material, instead of a regular scalpel during explantation [7].
The importance of education and increased practice of healthcare providers in the prevention of complications associated with catheter implantation has been noted in various guidelines [11,21]. It has been clearly shown that the inadequacy and inexperience of the nurses and ambulatory staff responsible for the care of central venous catheters are closely related to the increase in catheter-related infection and thrombosis [25]. Accordingly, the multimodal programs (education of home health nurses, oncology nurses and ambulatory staff) implemented in our hospital may have also had an effect on the decrease in TIVAP explantation rates due to infection and thrombosis over time [7]. In the study, it is thought that the reason why the in situ time of TIVAPs placed in the second period is longer than in the first period, is that the rates of TIVAP explantation due to this infection and thrombosis decrease over time. Again, in the periodic comparison, a decrease was found in the rate of early TIVAP explantation in the second period. The reason for this is thought to be the decrease in the rates of technical complications (dysfunction, etc.) that cause TIVAP explantation, mostly in the first 30 days. The decrease in these technical complications over time also shows us that physicians have a learning curve for the implantation process [1]. We can see another effect of this learning curve in the reduction of post-explant complications over time. Supporting our results, in a study by D’Souza et al [1], it was shown that the incidence of both complications and infections decreased significantly in the second study period, and attention was drawn to the learning curve of healthcare staff.
This study has several limitations. Firstly, the study is a single-center, non-randomized, retrospective study with relatively small sample size. Secondly, the study consisted of patients with different underlying malignancies and the use of different brands of catheters, therefore different chemotherapeutic regimens and variations in material quality may have affected outcomes, complications that may cause explantation, and the TIVAP in situ time. Thirdly, since a retrospective database was used over a 6-year period in the study, a detailed analysis of the changes in the patient care that was improved over time could not be performed. Finally, most of the TIVAPs in the study were inserted into the subclavian vein, and only a few were inserted into the internal jugular vein. The bias of these data may affect the observational results. Prospective studies with more controlled patient enrollment will help to eliminate these limitations in the future. Besides, the strength of our study is that all patients were operated on by the same surgeon in the same hospital using the same surgical technique.
In conclusion, infection is the cause of more than half of TIVAP explantations, which may interrupt chemotherapy in oncology patients. Pathogens isolated in cultures of infected patients are predominantly natural members of the skin flora. Although infection is the factor that most significantly decreases TIVAP survival, it rarely causes TIVAP explantation in the early period. In addition, it is important that processes such as TIVAP implantation, care and explantation (although it may seem like simple medical processes) are carried out in multidisciplinary centers and by experienced staff, as better outcomes are closely related to interdisciplinary collaboration, training of healthcare providers and increasing experience over time.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Ethics approval
Approval was obtained from the Local Ethics Committee for Non-Interventional Clinical Studies of Biruni University (protocol number: 2022-49-7164-E).
Authors’ contribution
HUÇ; conceptualized and designed the study, collected and analyzed data, revised the final version of the manuscript and wrote the paper.
Reference
1) D’Souza PC, Kumar S, Kakaria A, Al-Sukaiti R, Al-Baimani K, Hamid RS et al. Complications and Management of Totally Implantable Central Venous Access Ports in Cancer Patients at a University Hospital in Oman. Sultan Qaboos Univ Med J 2021; 21: 103-9.
2) Kılıç S, Soyer T, Karnak İ, Çiftçi AÖ, Tanyel FC, Şenocak ME. Evaluation of the removal reasons of totally implantable venous devices in children: a retrospective study. Turk J Pediatr 2016; 58: 187-94.
3) Biffi R, de Braud F, Orsi F, Pozzi S, Mauri S, Goldhirsch A et al. Totally implantable central venous access ports for long-term chemotherapy. A prospective study analyzing complications and costs of 333 devices with a minimum follow-up of 180 days. Ann Oncol 1998; 9: 767-73.
4) Ng F, Mastoroudes H, Paul E, Davies N, Tibballs J, Hochhauser D et al. A comparison of Hickman line- and Port-a-Cath-associated complications in patients with solid tumours undergoing chemotherapy. Clin Oncol 2007; 19: 551-6.
5) Di Carlo I, Cordio S, La Greca G, Privitera G, Russello D, Puleo S et al. Totally implantable venous access devices implanted surgically: a retrospective study on early and late complications. Arch Surg 200; 136: 1050-3.
6) Chang L, Tsai JS, Huang SJ, Shih CC. Evaluation of infectious complications of the implantable venous access system in a general oncologic population. Am J Infect Control 2003; 31: 34-9.
7) Fischer L, Knebel P, Schröder S, Bruckner T, Diener MK, Hennes R et al. Reasons for explantation of totally implantable access ports: a multivariate analysis of 385 consecutive patients. Ann Surg Oncol 2008; 15: 1124-9.
8) Shaul DB, Scheer B, Rokhsar S, Jones VA, Chan LS, Boody BA et al. Risk factors for early infection of central venous catheters in pediatric patients. J Am Coll Surg 1998; 18: 654-8.
9) Skummer P, Kobayashi K, DeRaddo JS, Blackburn T, Schoeneck M, Patel J et al. Risk Factors for Early Port Infections in Adult Oncologic Patients. J Vasc Interv Radiol 2020; 31: 1427-36.
10) Pandey N, Chittams JL, Trerotola SO. Outpatient placement of subcutaneous venous access ports reduces the rate of infection and dehiscence compared with inpatient placement. J Vasc Interv Radiol 2013; 24: 849-54.
11) O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control 2011; 39: 1-34.
12) Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999; 27: 97-132.
13) Schwarz RE, Groeger JS, Coit DG. Subcutaneously implanted central venous access devices in cancer patients: a prospective analysis. Cancer 1997; 79: 1635-40.
14) Uzunkaya F, Soylu Aİ, Belet Ü, Terzi Ö, Akan H. Reasons for removal of central venous ports: Experience with 154 consecutive patients. Ege Tıp Dergisi 2018; 57: 232-7.
15) Biacchi D, Sammartino P, Sibio S, Accarpio F, Cardi M, Sapienza P et al. Does the implantation technique for totally implantable venous access ports (TIVAPs) influence long-term outcome? World J Surg 2016; 40: 284-90.
16) Tabatabaie O, Kasumova GG, Eskander MF, Critchlow JF, Tawa NE, Tseng JF. Totally Implantable Venous Access Devices: A Review of Complications and Management Strategies. Am J Clin Oncol 2017; 40: 94-105.
17) Bamba R, Lorenz JM, Lale AJ, Funaki BS, Zangan SM. Clinical predictors of port infections within the first 30 days of placement. J Vasc Interv Radiol 2014; 25: 419-23.
18) Cesca E, Dall’igna P, Boscolo-Berto R, Meneghini L, Petris MG, Zanon GF et al. Impact of severe neutropenia and other risk factors on early removal of implanted central venous catheter (ICVC) in children with hematologic malignancies. J Pediatr Hematol Oncol 2014; 36: 541-4.
19) Tang L, Kim CY, Martin JG, Pabon-Ramos WM, Sag AA, Suhocki PV et al. Length of Stay Predicts Risk of Early Infection for Hospitalized Patients Undergoing Central Venous Port Placement. J Vasc Interv Radiol 2020; 31: 454-61.
20) Zhang S, Kobayashi K, Faridnia M, Skummer P, Zhang D, Karmel MI. Clinical Predictors of Port Infections in Adult Patients with Hematologic Malignancies. J Vasc Interv Radiol 2018; 29: 1148-55.
21) Gastmeier P, Geffers C. Prevention of catheter-related bloodstream infections: analysis of studies published between 2002 and 2005. J Hosp Infect 2006; 64: 326-35.
22) Goltz JP, Kickuth R, Scholl A, Machann W, Ritter CO, Hahn D et al. Explantation of totally implantable venous access ports of the forearm: reasons for removal and observed complications. J Vasc Access 2011; 12: 45-51.
23) Burzotta F, Romagnoli E, Trani C. Percutaneous removal of an embolized port catheter: description of a new coaxial recovery technique including a case-report. Catheter Cardiovasc Interv 2008; 72: 289-93.



