

2Department of Thoracic Surgery, University of Health Sciences, Dr. Suat Seren Chest Diseases and Surgery Medical Practice and Research Center, Turkey DOI : 10.26663/cts.2022.021
Summary
Background: Postpneumonectomy bronchopleural fistula is still one of the complications that are difficult and time-consuming to treat despite advances in surgical technique and technology. Although many surgical and non-surgical methods have been described in its treatment, there is no consensus on the optimal approaches. In this study, we aimed to share our experience in tracheobronchial stenting and to evaluate the efficacy and safety of the modified silicone stents and J-shaped endobronchial selfexpandable nitinol stent in patients with postpneumonectomy bronchopleural fistula presenting with severe dyspnea and massive air leak.Materials and Methods: We retrospectively analyzed data of the patients who underwent tracheobronchial stenting for postpneumonectomy bronchopleural fistula between January 2010 and December 2020 in our center. Two different endobronchial stent types were used. Modified silicone and covered self-expandable nitinol stent. The clinical features of the patients and the results of the intervention were evaluated.
Results: A total of 10 patients with bronchopleural fistulas were treated with tracheobronchial stenting. Modified silicone stent was used in 4, full covered J-shape nitinol stent was used in 6 patients. No complications were observed during the procedure. After the placement of the tracheobronchial stent, air leakage ceased in all patients and their dyspnea regressed significantly.
Conclusions: Tracheobronchial stents are an appropriate treatment option in patients with postpneumonectomy bronchopleural fistula presenting with severe dyspnea and massive air leak.
Introduction
Bronchopleural fistula (BPF) which is one of the most serious complications that can be seen after lung resections is a communication between the tracheobronchial tree and the pleural space. The incidence of BPF is 4.5% to 20% after pneumonectomy and 0.5% to 1% after lobectomy. Clinical presentation can range from a nonspecific chronic cough to serious clinical conditions such as empyema, pneumonia, and respiratory failure [1-3].Treatment includes pleural drainage and irrigation, surgically fistula repairment (with or without vascularized tissue transposition) and endoscopic approaches like stent implantation and tissue glue applications. Among the living tissue flaps, especially the omentum has been reported to be used in the prevention of BPF development, as well as in the treatment of fistula and infected pleural cavity. However, there is no consensus on the best treatment option and the treatment algorithm in patients with postoperative BPF. Undoubtedly, the clinical condition of the patient has a great influence on the choice of treatment method [4-7].
The use of bronchoscopic methods in BPF repair has always been an attractive alternative for the patient especially whose general condition is poor and cannot tolerate a major surgical intervention. However, patients with BPF are a very heterogeneous group in terms of their clinical status, fistula localization, and underlying etiologies therefore, subgroup studies are needed to create correct treatment algorithms.
Along with the heterogeneity in patient groups, endoscopic treatment methods also show a wide variety. Some of the endoscopic methods defined in the literature for closure of BPF are as follows: bronchial stents, tissue glue, coils, N-butyl cyanoacrylate glue, and bronchial occluders [8-15].
In this study, we aimed to present the results of endobronchial stenting in patients with postpneumonectomy bronchopleural fistula who have severe respiratory distress due to massive air leak.
Methods
The protocol of this study has been approved by the local ethics committee of our center. We retrospectively analyzed data of the lung cancer patients with postpneumonectomy bronchopleural fistula who underwent non-surgical fistula repairment between January 2010 and December 2020 in our center. Among these patients, those who underwent endobronchial stent placement with rigid bronchoscopy were included the study. Endobronchial stents were preferred in patients with severe dyspnea due to massive air leakage and who could not tolerate a major surgical intervention due to their comorbid conditions.BPF was diagnosed with flexible fiberoptic bronchoscopy, which performed on clinical suspicion in all patients. Chest computed tomography (CT) was carried out in all patients to evaluate the post-pneumonectomy cavity and contralateral lung. The chest tube was inserted in all patients and appropriate antibiotic therapy was administered.
Two different endobronchial stent types were used in patients: Modified silicon and covered self-expandable nitinol stent. The choice of stent to be used was determined by the localization of the fistula, the urgency of the patient’s clinical condition, and the availability of material. The preoperative and postoperative dyspnea of the patients were evaluated with the visual analog dyspnea scale (VADS).
Endobronchial treatment
All the procedures were carried out at the operation theatre under general anesthesia. Endobronchial stents were implanted via the rigid bronchoscopy.
We used 2 types of stents in our study; modified silicone stent and J-shaped self-expanding fully covered nitinol stents.
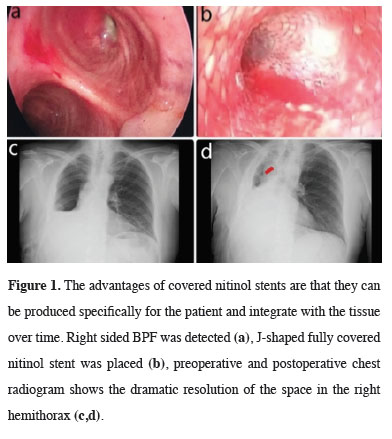
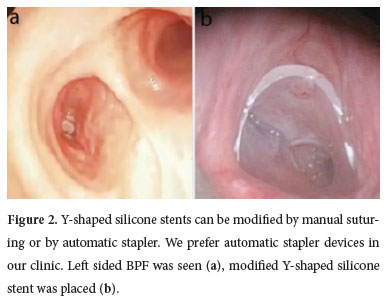
Silicone stents were modified with two different techniques. In the first technique, the straight silicone stent was made conical by dividing it with an automatic stapler device in accordance with the bronchial stump length (Figure 1). This technique was applied in only one patient who had a long bronchial stump and a small fistula size. In the other technique, Y-shaped endobronchial Dumon® stent (Novatech SA, La Ciotat, France) customized by closing the one side with an automatic stapler device (Figure 2). The length of the closed limb was determined by the measurement of the bronchial stump with chest CT and bronchoscopy. After the customization, the stent was placed with the NOVATECH® stent applicator through the rigid bronchoscope.
 Click Here to Zoom |
Figure 1: The advantages of covered nitinol stents are that they can be produced specifically for the patient and integrate with the tissue over time. Right sided BPF was detected (a), J-shaped fully covered nitinol stent was placed (b), preoperative and postoperative chest radiogram shows the dramatic resolution of the space in the right hemithorax (c,d). |
 Click Here to Zoom |
Figure 2: Y-shaped silicone stents can be modified by manual suturing or by automatic stapler. We prefer automatic stapler devices in our clinic. Left sided BPF was seen (a), modified Y-shaped silicone stent was placed (b). |
J-shaped endobronchial self-expandable nitinol stent (Aerstent® Trachea Bronchus Nitinol Stent; Leufen Medical GmbH, Berlin, Germany) was placed through its own ready-to-use applicator (Figure 3). The distal and proximal diameters and lengths of the stent lumen were adjusted specifically for the patients.
 Click Here to Zoom |
Figure 3: Modified straight silicone stent may be an alternative approach in patients with long bronchial stump and small fistula. One end of the straight silicone stent was stapled and cut according to the bronchial stump length (a), the modified silicone stent was placed through the rigid bronchoscope with the appropriate applicator (b), left sided BPF is seen (c), the appropriate length of silicone stent completely obliterated the fistula opening (d). |
Postoperative follow-up
After the operation patients were monitored for two hours in postoperative intensive care unit. In the postoperative day 1 chest X-ray, complete blood count, and C-reactive protein results were obtained. The antitussive medication (Oxolamine phosphate 150 mg t.i.d.) administered for seven days to prevent the displacement of the stent. Even if the air leak ended after endobronchial stent application, tube thoracostomy was not terminated in the early period. Two pleural culture negativity, observation of the stent in place in the bronchoscopic examination performed at the end of the first month, and the radiological observation that the postpneumonectomy space began to shrink were our indications for tube thoracostomy termination.
Patients who tolerated endobronchial stent well and did not require intravenous antibiotic treatment were discharged after 24 hours. At the end of the first week, the antibiotic treatment of the patients who were called for control was revised according to the secretion and pleural culture results. Routine bronchoscopic control was performed in the postoperative 1st month in all patients.
The primary outcome of this study the success of the stent in improving respiratory parameters in the early period. The visual analogue dyspnea scale (VADS) was used for measuring dyspnea before and after the stent implantation. The secondary outcome was the rate of BPF closure after the stent removal in long term follow-up.
Statistical Analyses
Statistical analysis was performed using the SPSS 25.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were presented as counts and percentages. Continuous variables were expressed as mean value ± standard deviation (SD). The change in variables over time was analyzed by paired t-test. Statistical significance was set at p-value < 0.05 (All p values presented were 2-sided).
Results
Between January 2010 and December 2020, a total of 243 patients with a diagnosis of lung cancer underwent pneumonectomy. Among these patients, postpneumonectomy BPF developed in 19 (7.8%) patients. 10 patients who underwent tracheobronchial stent in the management of BPF were included in the study. The mean age of the patients was 56.8 ± 8.0. The median time from lung resection to the diagnosis of bronchopleural fistula was 5.4 months (range 1-12). There were 7 (70%) patients with right pneumonectomy, 3 (30%) with left pneumonectomy. The mean BPF size was 9.6 ± 10.8 mm. The characteristics of the patients are summarized in the table 1.Table 1: Patient characteristics
Full covered J-shape nitinol stent was used in 6 (Figure 1), a modified silicon stent was used in 4 patients (a modified Y-shaped silicone stent was used in 3 (Figure 2), stapled straight silicone stent was used in 1 patient (Figure 3). There wasn’t seen any intraoperative complication. In the postoperative period stent migration was observed in one patient at the postoperative first month. The malposed stents were removed via rigid bronchoscopy. Stent occlusion due to granulation tissue and mucus plug was seen in 2 patients.
The mean dyspnea score measured by VADS was 7.10 ± 0.87 before the procedure and 4.30 ± 0.67 at the postoperative 1st week, and this difference was statistically significant (p < 0.001).
According to the long-term results, complete closure of the BPF was observed in 2 patients, and stents were terminated. Mortality due to disease progression was observed in 4 patients. In one patient tracheobronchial stent was terminated due to respiratory distress associated with stent malposition. Three patients with tracheobronchial stents are still being followed up.
Discussion
Because it is associated with high morbidity and mortality, BPF is one of the most undesirable complications after lung resections. Although it can accompany many other clinical conditions, it is often seen after pneumonectomy. Its incidence following pneumonectomy ranging from 0.8% up to 8.5% and mortality ranging from 16% to 72% [1-3]. In our study, the BPF rate in patients who underwent pneumonectomy was found to be 7.8%. The slightly higher rate compared to the literature was attributed to the sociocultural status of the patient population and low self-care, higher prevalence of comorbid conditions such as smoking, COPD, and tuberculosis.Many surgical and non-surgical methods have been described in the treatment of bronchopleural fistulae. The first option in the treatment of early BPF after pneumonectomy is surgical repair of the bronchial stump with reoperation. Since there is almost always an infection in the pleural cavity in late BPF, the priority in treatment is to control this pleural infection. Drainage of air and infected pleural fluid from the pleural space should be performed via the chest tube thoracostomy and broad-spectrum antibiotic therapy must be initiated. There is no consensus on the optimal curative treatment method that should be applied after the control of the pleural infection. In suitable patients, transsternal transpericardial bronchial stump repair, thoracomyoplasty, omentopexy, open drainage methods (eg. thoracostoma) can be applied separately or as combined treatments [4-8].
However, these patients are generally not in a position to handle a major surgical intervention due to their poor performance status and the presence of comorbid conditions. Tracheobronchial stents can improve the general condition and performance status of patients and eliminate the risk of aspiration of the pleural content to the contralateral lung.
The use of various stent types to closure of the BPF has been previously described in the literature [8-13]. Amaral et al [15] reported the use of a silicone Y-shaped endobronchial stent with a mechanically stapled distal limb to support the closure of a BPF. Similarly, de Lima et al [16] suggested manually modified endobronchial stents to closure of the BPFs. Han et al [17] describes the use of customized Y-shaped, L-shaped and hinged self-expandable covered metallic stent to closure post pulmonary resection BPFs.
In this study we used J-shaped fully covered nitinol metallic stent in 6 patients and mechanically stapled Y-shaped or straight silicon Dumon stent in 4 patients. The biggest advantages of covered nitinol stents are that they can be modified individually and adhere to the bronchial wall by expanding at body temperature. However, their costs are much higher than the silicon stents, and procure covered nitinol stents may take several days as they are produced on-demand. In addition, fractures may occur in nitinol metallic stents due to coughing and respiratory movements or in case of migration, therefore, unlike silicone stents, it is not possible to place them again.
The biggest disadvantage that draws our attention in silicone stents is that the lumen opening is severely narrowed by folding in the presence of deviation in the tracheobronchial system. Therefore, we recommend the use of fully covered self-expendable nitinol stents in patients with severe deviation of the tracheobronchial system towards the postpneumonectomy space.
Placement of a stapled straight silicone stent is a new technique that was reported for the first time in the literature by Amaral et al [15]. We think that this technique would be more appropriate in patients with long bronchial stumps and small fistulas due to the lower risk of stent migration. For this reason, we applied it to only one patient who met these criteria and we achieved a successful result.
Several stent-related complications were described in the literature. The most common of these complications are stent migration, excessive granulation tissue, secretion retention, stent fracture and poor patient tolerance [8-10,16-20].
In long term follow-up stent migration was seen in one patient with covered nitinol stent. The malposed stent was removed via rigid bronchoscope. Because fistula size decreased it was decided to follow the patient without a stent. The most seen long-term complication in our study was granulation tissue. Granulation tissue was observed in all patients who underwent stenting, but it was enough to narrow the airway in only 2 patients. In these patients, one with a silicone and the other with a covered nitinol stent, electro-cauterization was performed via rigid bronchoscopy.
Unfortunately, we have not been able to terminate the thorax drainage tubes permanently among these patients. Short survival time and recurrent empyema are among the reasons for this condition. Bacterial colonization of the stents and chronic contamination of the pleural space may facilitate the empyema occurrence. However, in our study, we do not have any data to prove this pathogenesis.
Because of the many potential stent-related complications mentioned above, we do not recommend endobronchial stenting as a routine treatment for postpneumonectomy BPFs. The general condition of the patient, survival expectancy and BPF occurrence time should be considered in the selection of the appropriate treatment method.
In conclusion tracheobronchial stents are useful in eliminating the risk of aspiration of infected pleural contents and reducing respiratory distress in patients with massive air leak. We suggest tracheobronchial stents in patients with late BPF that cause massive air leak, as they reduce respiratory distress and prevent aspiration of infected pleural contents. Depending on the general condition of the patient and the structure of the tracheobronchial system, nitinol or silicone stents may be preferred.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Ethics approval
This study was approved by the local ethics committee of Health Sciences University Dr. Suat Seren Chest Diseases and Chest Surgery Training and Research Hospital (approval number: 49109414-604.02).
Authors’ contributions
KCC,SOK; conceptualized and designed the study, collected analyzed and interpreted the patient data regarding the pulmonary metastasis, co-wrote the paper, GB; collected and analyzed data, revised the final version of the manuscript and wrote the paper. All authors read and approved the final manuscript.
Reference
1) Boudaya MS, Smadhi H, Zribi H, Mohamed J, Ammar J, Mestiri T et al. Conservative management of postoperative bronchopleural fistulas. J Thorac Cardiovasc Surg 2013; 146: 575-9.
2) Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Semin Thorac Cardiovasc Surg 2001; 13: 3-7.
3) Farkas EA, Detterbeck FC. Airway complications after pulmonary resection. Thorac Surg Clin 2006; 16: 243-51.
4) Bribriesco A, Patterson GA. Management of postpneumonectomy bronchopleural fistula: from thoracoplasty to transsternal closure. Thorac Surg Clin 2018; 28: 323-35.
5) de la Riviere AB, Defauw JJ, Knaepen PJ, van Swieten HA, Vanderschueren RC, van den Bosch JM. Transsternal closure of bronchopleural fistula after pneumonectomy. Ann Thorac Surg 1997; 64: 954-7. discussion 958-9.
6) Ulusan A, Benli MY, Ekici MA, Sanlı M, Isık AF. Omentum Transposition as a Solution for Bronchopleural Fistula and Empyema. Indian J Surg 2020; 82: 74-80.
7) Sarkar P, Chandak T, Shah R, Talwar A. Diagnosis and management of bronchopleural fistula. Indian J Chest Dis Allied Sci 2010; 52: 97e102.
8) Tsukada H, Osada H. Use of a modified Dumon stent for postoperative bronchopleural fistula. Ann Thorac Surg 2005; 80: 1928-30.
9) Fruchter O, El RBA, Abdel-Rahman N, Saute M, Bruckheimer E, Kramer MR. Efficacy of bronchoscopic closure of a bronchopleural fistula with amplatzer devices: long-term follow-up. Respiration 2014; 87: 227-33.
10) Cao M, Zhu Q, Wang W, Zhang TX, Jiang MZ, Zang Q. Clinical application of fully covered self-expandable metal stents in the treatment of bronchial fistula. Thorac Cardiovasc Surg 2016; 64: 533-9.
11) Tayama K, Eriguchi N, Futamata Y, Harada H, Yoshida A, Matsunaga A et al. Modified Dumon stent for the treatment of a bronchopleural fistula after pneumonectomy. Ann Thorac Surg 2003; 75: 290-2.
12) Toa H, Araki M, Sato T, Morino S, Kawanami R, Yoshitani M et al. Bronchoscopic treatment of postpneumonectomy bronchopleural fistula with a collagen screw plug. J Thorac Cardiovasc Surg 2006; 132: 99-104.
13) Watanabe Y, Matsuo K, Tamaoki A, Komoto R, Hiraki S. Bronchial occlusion with endobronchial Watanabe spigot. J Bronchol 2003; 10: 264-7.
14) Mehta HJ, Malhotra P, Begnaud A, Penley AM, Jantz MA. Treatment of alveolar-pleural fistula with endobronchial application of synthetic hydrogel. Chest 2015; 147: 695-9.
15) Amaral B, Feijó S. Fistula of the Stump: A Novel Approach With a “Stapled” Stent. J Bronchology Interv Pulmonol 2015; 22: 365-6.
16) de Lima A, Holden V, Gesthalter Y, Kent MS, Parikh M, Majid A et al. Treatment of persistent bronchopleural fistula with a manually modified endobronchial stent: A case-report and brief literature review. J Thorac Dis 2018; 10: 5960-3.
17) Han X, Yin M, Li L, Zhu M, Ren K, Qi Y et al. Customized airway stenting for bronchopleural fistula after pulmonary resection by interventional technique: single-center study of 148 consecutive patients. Surg Endosc 2018; 11: S1274-6.
18) Zeng J, Wu X, Chen Z, Ke M. Modified silicone stent for the treatment of post-surgical bronchopleural fistula: a clinical observation of 17 cases. BMC Pulm Med 2021; 21: 10.



