

2Department of Thoracic Surgery, Ankara City Hospital, Ankara, Turkey
3Department of Thoracic Surgery Ataturk Chest Diseases and Thoracic Surgery Training and Research Hospital, Ankara, Turkey DOI : 10.26663/cts.2022.022
Summary
Background: Although rare, thymomas are common primary tumors of the anterior mediastinum. Herein we aimed to investigate the outcomes of thymoma surgeries, with a focus on survival rates and in reference to the demographic and histological characteristics of patients. The secondary aim is to identify the factors that affect recurrence.Materials and Methods: Fifty-five patients were operated on for thymoma have been retrospectively evaluated according to their demographics, clinical characteristics, pathologies, complications, recurrences and survival.
Results: The mean SUVmax value was found to be 5.5 ± 2.05 and showed no correlation with mass diameter or stage (p = 0.284 and p = 0.176, respectively). The mean survival was 55.9 ± 35.31 months in the R0 resection group. Overall survival was not correlated with age and mass diameter at a statistically significant level (p = 0.056, p = 0.108 respectively). There was no difference in the frequency of recurrence between the WHO stages (p = 0.775). Conversely, when classified as per the Masaoka–Koga classification, recurrence was detected in all stage-4 patients (p < 0.001).
Conclusions: To date, there is no practical structure that classifies and integrates prognostic factors and creates a usable system out of them but, in thymomas, the best results are achieved by complete surgical resection.
Introduction
Thymomas originating from the epithelial cells of the thymus are the most common neoplasms of the anterior mediastinum. There have been some confusion regarding the staging of thymomas and their treatment management since they are encountered rarely. Over the last 50 years, various classifications of thymomas have been reported based on the content of non-neoplastic lymphocytes accompanying malignant epithelial cells of the thymus, the invasiveness of the tumor, and its localization in the thymus [1].Invasiveness is the most important prognostic factor, and surgery remains to be the first choice in the treatment of thymomas at any stage where complete resection (R0) is attainable. The basic principle of thymus surgery is a full exploration of the mediastinum, en-bloc resection of the tumor and adjacent mediastinal adipose tissue, preservation of the phrenic nerves, and prevention of intrapleural spread. The surgical options in thymectomy include partial or median sternotomy, thoracotomy, video-assisted thoracic surgery (VATS), and robot-assisted surgery [2]. Including the thymoma surgeries performed in a single center during 12 years, the present study primarily intended to investigate the outcomes of these surgeries, with a focus on survival rates and about the demographic and histological characteristics of patients. The secondary aim is to identify the factors that affect recurrence.
Methods
Patient population and objectivesAfter approval from the local ethics committee of Ataturk Chest Diseases and Thoracic Surgery Training and Research Hospital (551/2017) we conducted a retrospective investigation of 55 patients with thymoma who underwent surgery for anterior mediastinal mass at the Ataturk Chest Diseases and Thoracic Surgery Training and Research Hospital Thoracic Surgery Clinic between January 2005 and December 2016 and whose postoperative pathology result indicated thymoma. Patients with thymic hyperplasia and carcinomas were excluded from the study.
Patients’ age, gender, symptoms, smoking history, and comorbidities; the type of operation; the presence of myasthenia gravis (MG); thymic pathology; the length of hospital stay; drain duration; mass diameter; histological and clinical stage; maximum standard uptake value (SUVmax); the administration of radiotherapy and/or chemotherapy; complications; recurrence; and survival were recorded. Only VATS biopsy and no further resection were performed in 3 of the 55 cases. In 3 patients who underwent VATS with the goal of complete resection, perioperatively, it was understood that complete surgical resection could not be performed so only biopsy was performed. Survival according to surgical type and resection status was calculated on a total of 52 patients, excluding these three.
Pathological staging was performed in accordance with the staging criteria of both Masaoka–Koga and the World Health Organization (WHO). When staging was performed as per the WHO classification, the advanced component in mixed-type thymomas was taken as the basis.
Postoperative complications observed within the first 30 days were defined as early complications. Charlson comorbidity index was used to define and grade comorbidities [3]. As per this index, comorbid diseases are scored according to their severity. Comorbidities were given a score of 1–4 for mild–severe disease, and comorbidity was graded according to the weighted score obtained by summing the scores of comorbid diseases.
Preoperative evaluation and intraoperative indications
Posteroanterior and lateral X-ray imaging of the chest and computed tomography (CT) were performed preoperatively in all the patients. Routine blood and pulmonary function test results of the patients were also examined. Additionally, 27 (49%) patients were also examined using positron emission tomography (PET)-CT. All patients with a history of MG were first examined by neurologists before the administration of anesthesia, and then the treatment that was deemed appropriate for them was administered accordingly.
Surgical technique and follow-up
In all cases wherein total resection was attainable, in addition to the resection of the mass, with the surgical techniques performed (median-sternotomy, thoracotomy, VATS), the thymic vessels were also ligated. Additionally, all tissues that may belong to the thymus, which were traced up to the diaphragm along the course of the phrenic nerve, were removed along with the mediastinal adipose tissues. If the mass had invaded the surrounding lung tissues, wedge resection was performed to resect it. Furthermore, observable pleural metastases were excised completely.
The postoperative pathology results of all the patients were evaluated by medical and radiation oncology specialists. A therapy regimen was planned for the patients in whom chemotherapy and/or radiotherapy was deemed necessary. After routine follow-ups on the 15th postoperative day and 3rd postoperative month, the patients were followed up with chest radiography every 3 months for up to 1 year and once every year thereafter. Patients in whom clinical and radiological suspicion of recurrence arose were examined using chest CT.
Statistical Analysis
Data were analyzed using the IBM SPSS Statistics statistical package program. The descriptive variables are presented as number of units (n), percentage (%), mean ± standard deviation (x ̅±ss), and median (Q1–Q3) values. To analyze the categorical variables, Pearson’s chi-square and Fisher’s exact tests were used. Shapiro–Wilk test and Q–Q plots were used for checking whether the numerical variables had a normal distribution. To compare the two groups, independent samples t-test and Mann–Whitney U analysis were performed for normally and non-normally distributed variables, respectively. The Kaplan–Meier method was used for survival analysis. Intergroup comparison of survival was performed using the log-rank test. To determine the correlation between two numerical values, Pearson’s and Spearman’s correlation analyses were performed for normally and non-normally distributed variables, respectively. The statistical significance level was set at p ≤ 0.05.
Results
The age range of the patients was 10-73 years, and the median age was 48.7 years. In our study, 27.2% of the patients (n =15) were in their 50s and 20% (n =11) were in their 60s; these patients accounted for the majority of the study population. Furthermore, 24 (43.6%) patients were female and 31 (56.4%) were male.Preoperative fine needle aspiration biopsy was performed for 15 (27.2%) patients. While 12 patients were diagnosed with thymoma, the remaining 3 patients were reported to have neurogenic tumor, thymic carcinoma, and lymphoid hyperplasia; this did not match with the postoperative pathological diagnosis.
The demographic and clinical characteristics of the patients as well as their stages classified as per the Masaoka–Koga and WHO classifications are shown in table 1.
Table 1: Demographic, clinical and histological char-acteristics of the patient.
Median sternotomy was performed in 32 cases, posterolateral thoracotomy was performed in 15 cases and VATS was performed in 5 cases. The median length of hospital stay and the median drain duration were shown in table 2.
Table 2: The median length of hospital stays and the median time of drain duration.
For 27 patients who underwent PET/CT, the mean SUVmax value was found to be 5.5 ± 2.05 and showed no correlation with mass diameter or stage (p = 0.284 and p = 0.176, respectively).
During the postoperative period, 10 (18.1%) patients received chemotherapy, 22 (40%) received radiotherapy, and 9 (16.3%) patients received both chemo and radiotherapy. While there were 13 patients (23.6%) who received radiotherapy alone. The mean follow-up period of the patients was 52.5 months.
There were three early complications in our study, and all of them were encountered in patients who underwent median sternotomy. Of these patients, sternal dehiscence developed in one patient and wound infection in another patient. Clinical improvement was achieved via wound debridement and meticulous wound care. Furthermore, postoperative pneumonia developed in one patient. Rapid clinical response was also achieved in this patient with antibiotic treatment. In our study, no early or late complications were observed in the VATS and thoracotomy group patients. In terms of the incidence of complications, there was no significant difference between the patients who received and those who did not receive radiotherapy and between the patients who received and those who did not receive chemotherapy (p = 0.163, p < 1.000). Additionally, the number of female and male patients did not differ in terms of their rates of incidence of complications (p = 0.314).
In our study, a total of ten patients died during the follow-up period. As per the Masaoka–Koga classification, one patient was in stage 1, two were in stage 2a, one was in stage 2b, four were in stage 3, and two were in stage 4a. One patient died in the early period due to postoperative myocardial infection, whereas another patient died due to respiratory failure secondary to massive pulmonary embolism in the 2nd postoperative month.
It was concluded that overall survival was not correlated with age and mass diameter at a statistically significant level (p = 0.056, p = 0.108 respectively).
Survival according to resection status
The mean survival was 55.9± 33.38 months in the R0 resection group (n = 43), 32.4 ± 22.44 months in the R1 resection group (n = 7), and 57 ± 23.45 months in the R2 resection group (n = 2). Considering the resection status, the R0 and R2 resection groups were found to differ from the R1 resection group at a statistically significant level in terms of survival (p = 0.005) (Figure 1).
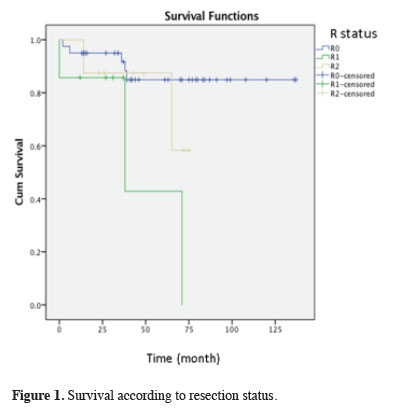 Click Here to Zoom |
Figure 1: Survival according to resection status. |
Survival according to WHO and Masaoka–Koga classifications
The overall survival of all the cases was found to be 52.49 months. When evaluated as per WHO classification, there was no significant difference between the groups in terms of overall and mean life expectancies. In contrast, when evaluated as per the Masaoka–Koga classification, there was a significant difference between the groups in terms of overall and mean survivals. The stage 1 patient group was found to significantly differ from the stage 3 and stage 4 patient groups (p < 0.001, both). The stage 2B patient group was also found to significantly differ from the stage 3 and stage 4 patient groups (p = 0.015, p = 0.004) (Figure 2).
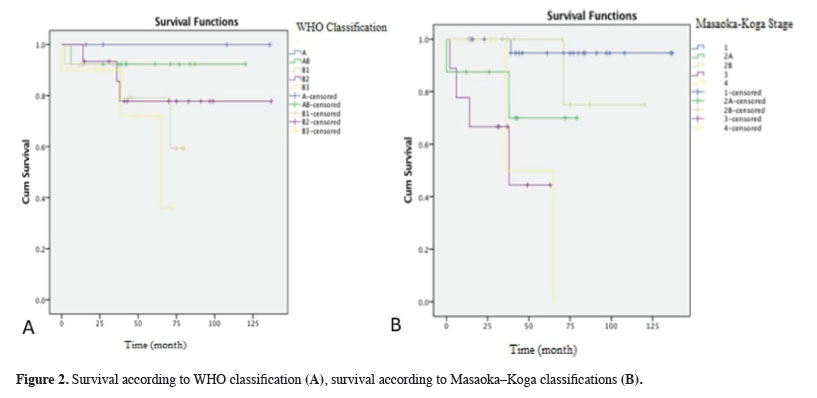 Click Here to Zoom |
Figure 2: Survival according to WHO classification (A), survival according to Masaoka–Koga classifications (B). |
Survival according to chemotherapy and radiotherapy statuses
The mean survival was 31.5 ± 19.87 months in the group of patients who received chemotherapy (n = 10), whereas it was 57.16 ± 34.12 months in those who did not receive chemotherapy (n = 45). While the mean survival was 38.45 ± 22.91 months in the group of patients who received radiotherapy (n = 22), it was 61.85 ± 36.19 months in those who did not receive radiotherapy (n = 33). There was a significant difference in overall and mean survivals between the patients who received and those who did not receive postoperative chemotherapy (p = 0.002). Additionally, there was a significant difference in overall and mean survivals between the patients who received and those who did not receive postoperative radiotherapy (p < 0.001).
Recurrence rates according to the resection status
Recurrence was detected in 20% of the cases (n = 11). Recurrence rates according to the resection status are given in figure 2. When classified as per the WHO classification, there was no difference in the frequency of recurrence between the stages (p = 0.775). Conversely, when classified as per the Masaoka–Koga classification, recurrence was detected in all stage-4 patients (n = 3) (p < 0.001).
Discussion
Thymomas are relatively poorly understood malignancies due to their relative rarity and lack of cumulative data. Approximately 30% of patients with thymoma have MG [4]. The patients with MG accounted for 10.9% of the study population. The relationship between smoking history and thymoma has not been proven; however, 38.1% of the patients in our study had a history of smoking.Preoperative diagnosis is not necessary for small, resectable tumors located in the anterior mediastinum that present with radiological features of typical thymoma. However, an interventional diagnostic procedure is required primarily in cases where nonoperative techniques or preoperative chemoradiotherapy is considered to be a better treatment modality or in patients with a high probability of having lymphoma. Fine needle aspiration biopsy has been reported to have a diagnostic success rate of 60% [5]. The diagnostic success rate in our study group was calculated to be 80%.
Although there are few reports on the utility of PET/CT in the diagnosis of thymic epithelial tumors, the number of patients in all these reports does not exceed 50. In our study, when patients were classified according to the WHO classification, the highest SUVmax value was found in patients with type B3 thymoma (mean SUVmax value; 7.52 ± 2.84); however, no significant difference was found between the groups. In contrast, when patients were classified according to the Masaoka–Koga classification, the highest SUVmax value was found in patients with stage 3 disease (mean SUVmax value; 7.84 ± 3.57). In a 11-disease study by Nakajo et al, the researchers grouped patients with thymomas of type A, AB, and B1 in the low-risk group and those with thymomas of type B2, B3, and thymic carcinoma in the high-risk group and found that the SUVmax value was significantly higher in the high-risk group [6,7]. In our study, the mean SUVmax value was found to be 5.5 regardless of the mass diameter and disease stage.
In a study comparing VATS and open thymectomy, the group of patients who underwent VATS had short operative time, length of hospital stay, and drain duration and a lower rate of complications [8]. In our study, we did not encounter any complications in the VATS group patients. Consistent with the literature, the length of hospital stay and drain duration were shorter in the VATS group than in the open thoracotomy and median sternotomy groups (p = 0.030). In recent years, robot-assisted thoracoscopic surgery (RATS) has been increasingly performed for thymectomy. In a study comparing RATS, VATS, and sternotomy, it was underlined that intraoperative blood loss was significantly lower and drain duration and length of hospital stay were significantly shorter in the RATS group, whereas the sternotomy group was found to have the highest rate of complications [9].
It is extremely difficult to obtain reliable results from the small biopsies performed to establish the histological subtype of thymoma due to the wide variation in the characteristic morphological presentations of thymomas [10]. The WHO classification provides further insight in understanding thymomas at an advanced level and recommends specific subtypes for varying clinical characteristics encountered in patients. However, they are still not fully reliable in deciding the optimal treatment. It is now more clearly understood that type C (thymic carcinoma) is a distinct group and its prognosis is quite different from the others. Moreover, prognosis varies also among the other subtypes (A, AB, and B1–3) [11]. Histological classification is primarily used to distinguish thymic carcinoma from others.
The presence of multiple classification schemes and the variability among observers in determining the histological stage constitute another set of problems. Additionally it has yet to be clarified which subtype caused the difference. The outcomes obtained for patients in the A, AB, and B1 stages were found to be better than those obtained for patients in the B2 and B3 stages [12]. Nonetheless, based on the existing knowledge that we have, it would still be wise to accept the stage as a valid prognostic factor in thymoma.
Five-year survival rates are quite good even for stage 3 and 4 patients. 10-year survival rates for stage 1, 2, 3, and 4a are reported to be approximately 90%, 70%, 55%, and 35%, respectively [13]. In our study, the lowest rate of overall survival was observed in type B3 tumors (70%) classified according to the WHO classification and in stage 4 tumors (33.3%) classified according to the Masaoka–Koga classification; however, no significant difference was observed. This result can be attributed to the small number of the cases included in our study. Furthermore, the group of patients with stages classified according to the Masaoka–Koga classification was found to have significant differences in terms of recurrence.
Adjuvant therapies are not recommended for stage 1 thymomas that have been fully resected. Postoperative radiotherapy is recommended for patients with unresectable masses, incomplete resections, and those with capsular invasion following R0 resection [14]. In our study, 13 patients were administered radiotherapy because of microscopic capsule invasion that occurred following R0 resection. The risk of postoperative recurrence is high in stage 3 thymomas; therefore, radiotherapy is recommended in such cases [15]. In our study, there were nine patients in the stage 3 group, and seven of them (77.7%) received postoperative radiotherapy. One patient could not receive radiotherapy due to sternal dehiscence, whereas the remaining one patient died due to postoperative myocardial infarction and, therefore, could not receive radiotherapy. There are publications stating that postoperative radiotherapy does not offer benefits in stage 2 thymomas [14,16]. In our study, 10 (45.4%) of the 22 patients who received radiotherapy had stage 2 thymoma, and recurrence was observed in only one of these patients.
Preoperative induction chemotherapy is recommended to achieve R0 resection in patients who are thought to suitable for surgery [17]. In patients with solitary or ipsilateral pleural metastases, induction chemotherapy or surgery is among the available options. In cases where tumors are unresectable, administering both chemotherapy and radiotherapy is recommended [16]. In our study, worse outcomes were observed in the groups receiving chemotherapy or radiotherapy, and this could be attributed to the stage and not the treatment. Therefore, this result cannot be interpreted to conclude that chemotherapy or radiotherapy has negative effects on survival.
Age is thought to affect survival to a lesser extent [18]. Our study concluded that age-related survival did not differ significantly between the studied groups. Additionally, it has been reported in the literature that age has no effect on recurrence [19]. In our study as well, no significant difference was found among the patients when their recurrence rates were compared according age (p = 0.424). If age does not have an effect on recurrence, it is not surprising that older patients have worse survival due to other comorbidities. In conclusion, it would be more accurate to consider that age is not a valuable prognostic factor.
Complete resection is actually the main factor whose prognostic importance has been proven in terms of survival and recurrence; however this parameter can only be assessed in patients who have undergone surgery. Many multivariate analyses have established that R0 resection is an independent prognostic factor [9]. In our study, since the patients in the R1 group died due to causes unrelated to thymoma, the R1 resection group was found to have a shorter survival rate than the R2 resection group.
In current study, recurrence was detected in all patients who underwent R2 resection, but no significant correlation was found between resection status and survival. The biggest shortcoming of Masaoka–Koga classification is the lack of a significant difference between patients who undergo complete resection and those who undergo incomplete resection of advanced tumors. However, there are studies in which high 5-year survival rates have been attained with extensive resection and reconstruction in operated patients, even in those in the advanced stages [9].
After primary surgical treatment, the patients with resectable thymoma should be followed up using chest CT scans performed once every 6 months for the first 2 years and then once a year for the next 10 years [20]. There are limited publications regarding the duration and frequency of follow-up and the radiological method that should be utilized in patients with thymoma. It is known that these patients have a high risk of developing secondary malignancies; however, there is no study that recommends performing screenings [12].
Since overall survival can be practically measured, it has been used in most cases to evaluate the surgical outcomes of thymic malignancies. However, it is not an ideal measurement because most patients with thymoma die due to causes unrelated to the disease and live for many years without recurrence. Recurrence is considered to be more useful as a parameter for assessing outcomes; however, it does not always cause death [18]. It seems more logical not to equate death with recurrence since death might occur due to any cause. Few studies have focused on recurrence and this is a field that definitely needs further research. [18-23]. In this study, recurrence was detected in a total of 11 patients (20%). The highest rate of recurrence was observed in type B2 tumors (26.7%) classified according to the WHO classification and in stage 4 patients (100%) classified according to the Masaoka–Koga classification.
This study has several limitations. First, it is a retrospective single-center study that is prone to selection bias and the sample size of the study is small. We also have limited postoperative follow-up information. Comparing the patients who received only chemotherapy and only radiotherapy with those who received chemoradiotherapy will provide valuable information by creating a more homogeneous patient group. The patients will be observed to see if recurrence will develop in the following years. Since robot-assisted surgery was not performed in any of the patients, we could not get the opportunity to compare this surgical method with the others.
In conclusion, there is an increasing need to predict the outcomes of patients with thymoma. When making clinical decisions regarding thymoma, completeness of the resection is primarily more valuable than the histological stage. The available data show that disease stage and complete resection are valuable prognostic factors. Furthermore, gender and presence of MG have been reported to have no prognostic value. The effect of tumor size and histology on prognosis should be investigated in future studies.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Ethics approval
Approval was obtained from the local ethics committee of Ataturk Chest Diseases and Thoracic Surgery Training and Research Hospital, Ankara, Turkey (protocol number: (551/2017)).
Authors’ contribution
MSI; conceptualization, designed the study, collected the data, contributed data analysis and wrote the paper. KI; collected the data and performed the analysis. SSEG; edited visualization, revised the final version of the manuscript. KA; edited visualization and data presentation. PB; revised the final version of the manuscript. GF; formulated and evaluated research goals. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work
Reference
1) Markowiak T, Hofmann HS, Ried M. Classification and staging of thymoma. J Thorac Dis 2020; 12: 7607-12.
2) Detterbeck F, Parsons A. Thymic tumors: a review of current diagnosis, clasification, and treatment in Thoracic and Eosphageal Surgery, 3rd ed., Philadelphia: Elsevier, 2008, p. 1589–614.
3) Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373-83.
4) Verley JM, Hollmann KH. Thymoma. A comparative study of clinical stages, histologic features, and survival in 200 cases. Cancer 1985; 55: 1074-86.
5) Herman SJ, Holub RV, Weisbrod GL, Chamberlain DW. Anterior mediastinal masses: utility of transthoracic needle biopsy. Radiology 1991; 180: 167-70.
6) Nakajo M, Kajiya Y, Tani A, Yoneda S, Shirahama H, Higashi M et al. ¹⁸FDG PET for grading malignancy in thymic epithelial tumors: significant differences in ¹⁸FDG uptake and expression of glucose transporter-1 and hexokinase II between low and high-risk tumors: preliminary study. Eur J Radiol 2012; 81: 146-51.
7) Liu RS, Yeh SH, Huang MH, Wang LS, Chu LS, Chang CP et al. Use of fluorine-18 fluorodeoxyglucose positron emission tomography in the detection of thymoma: a preliminary report. Eur J Nucl Med 1995; 22: 1402-7.
8) Pennathur A, Qureshi I, Schuchert MJ, Dhupar R, Ferson PF, Gooding WE et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011; 141: 694-701.
9) Qian L, Chen X, Huang J, Lin H, Mao F, Zhao X et al. A comparison of three approaches for the treatment of early-stage thymomas: robot-assisted thoracic surgery, video-assisted thoracic surgery, and median sternotomy. J Thorac Dis 2017; 9: 1997-2005.
10) Moran CA, Suster S. On the histologic heterogeneity of thymic epithelial neoplasms. Impact of sampling in subtyping and classification of thymomas. Am J Clin Pathol 2000; 114: 760-6.
11) Detterbeck FC. Clinical value of the WHO classification system of thymoma. Ann Thorac Surg 2006; 81: 2328-34.
12) Yamakawa Y, Masaoka A, Hashimoto T, Niwa H, Mizuno T, Fujii Y et al. A tentative tumor-node-metastasis classification of thymoma. Cancer 1991; 68: 1984-7.
13) Regnard JF, Magdeleinat P, Dromer C, Dulmet E, de Montpreville V, Levi JF et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996; 112: 376-84.
14) Forquer JA, Rong N, Fakiris AJ, Loehrer PJ Sr, Johnstone PA. Postoperative radiotherapy after surgical resection of thymoma: differing roles in localized and regional disease. Int J Radiat Oncol Biol Phys 2010; 76: 440-5.
15) Hassan M, Seoud DE. Multimodality treatments in locally advanced stage thymomas. Hematol Oncol Stem Cell Ther 2009; 2: 340-4.
16) Kondo K. Optimal therapy for thymoma. J Med Invest 2008; 55: 17-28.
17) Shamji F, Pearson FG, Todd TR, Ginsberg RJ, Ilves R, Cooper JD. Results of surgical treatment for thymoma. J Thorac Cardiovasc Surg 1984; 87: 43-7.
18) Huang J, Detterbeck FC, Wang Z, Loehrer PJ. Standard outcome measures for thymic malignancies. Zhongguo Fei Ai Za Zhi 2014; 17: 122-9.
19) Wright CD, Wain JC, Wong DR, Donahue DM, Gaissert HA, Grillo HC eta al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg 2005; 130: 1413-21.
20) Pan CC, Chen PC, Wang LS, Chi KH, Chiang H. Thymoma is associated with an increased risk of second malignancy. Cancer 2001; 92: 2406-11.
21) Cowen D, Richaud P, Mornex F, Bachelot T, Jung GM, Mirabel X et al. Thymoma: results of a multicentric retrospective series of 149 non-metastatic irradiated patients and review of the literature. FNCLCC trialists. Fédération Nationale des Centres de Lutte Contre le Cancer. Radiother Oncol 1995; 34: 9-16.



