

2Department of Thoracic Surgery, Cukurova University Faculty of Medicine, Adana, Turkey
3Department of Public Health, Cukurova University Faculty of Medicine, Adana, Turkey DOI : 10.26663/cts.2022.024
Summary
Background: There are limited data in the literature regarding the early and long-term results of surgery in non-cystic fibrosis bronchiectasis. The aim of this study is to contribute to literature with clear analysis of early postoperative complications and to determine the rate of postoperative complications in non-cystic fibrosis bronchiectasis.Materials and Methods: One hundred two patients with non-cystic fibrosis bronchiectasis who underwent surgical resection participated in the study between April 2012 and November 2019 at Çukurova University. Patients were contacted by phone and scheduled for a face-to-face interview. For five patients who died during the postoperative period, mandatory information was collected through family members or from retrospective assessment of their medical records.
Results: Of the 102 patients, 47 (46.1%) were male and 55 (53.9%) were female, with an average age of 38.8 ± 14.0 years. The indication for surgery was hemoptysis in 16 (15.7%), recurrent pulmonary infections in 74 (72.5%), and suspicion of malignant transformation in 12 (11.8%) patients. The mean follow-up period was 71.4 ± 28 months. The early postoperative complication rate was 21.5% with no intraoperative fatality and a 4.9% mortality rate with long-term follow-up. The most common postoperative complications were surgical site infection and hemorrhage. The type of bronchiectasis (varicose bronchiectasis), lower forced vital capacity (FVC) and the diffusing capacity of the lung for carbon monoxide (DLCO) in the perioperative period were identified as risk factors for the development of postoperative complications. Long-term mortality was also higher in individuals who had early postoperative complications.
Conclusion: With low mortality and morbidity rates, surgical interventions in non-cystic fibrosis bronchiectasis remains a feasible choice in selected cases.
Introduction
Bronchiectasis is a chronic lung illness marked by a vicious cycle of airway infection and inflammation that results in irreversible structural damage to the small airways and occasionally the surrounding lung tissue [1]. Although pulmonary rehabilitation, proper immunization, and microbial eradication therapy are the most significant treatments for bronchiectasis, alternative experimental treatment options and surgery are also useful in certain circumstances. The goal of surgical bronchiectasis therapy is to interrupt the vicious loop of bronchiectasis by removing non-functional lung segments and preventing contamination of surrounding lung zones. The most prevalent grounds for surgery are recurrent infections with persistent symptoms such as productive cough, purulent sputum, and hemoptysis [2,3].The prevalence of bronchiectasis has been estimated at 53-566/100,000 population as the prevalence increases with age and the female gender [4-8]. The age-adjusted mortality rate for bronchiectasis has been reported at 1437.7/100,000 population [7]. Several longitudinal studies have described up to a 30% mortality at 1-year follow-up after suffering an exacerbation, particularly in the presence of chronic obstructive pulmonary disease [9-11]. Surgical therapies for adult patients with bronchiectasis are not recommended, with the exception of individuals with localized illness and a high exacerbation frequency, even if all other elements of their bronchiectasis therapy have been optimized [12]. Operative mortality with curative surgery, on the other hand, is nearly 0%-1.4%, and the risk of postoperative complications is roughly 15%-16.2% [2,13]. Moreover, 71.5% of the adult population who underwent surgery reported complete symptom relief, whereas 20.2% reported a reduction in preoperative symptoms [2].
There is limited data in the literature regarding the early and long-term results of surgery in non-cystic bronchiectasis. The aim of this study is to contribute to the literature with clear analysis of early postoperative complications and to determine the rate of postoperative complications associated with surgery of non-cystic fibrosis bronchiectasis. The secondary outcomes include the indications for the surgical decision, the long-term symptoms after surgery, and long-term mortality after non-cystic fibrosis bronchiectasis surgery.
Methods
Study design and data collectionThis research is a cross-sectional study and includes a retrospective review of medical records with the approval of the ethical committee of Çukurova University. Between April 2012 and November 2019, 102 patients with non-cystic fibrosis bronchiectasis who underwent surgical resection participated in the study. All of the patients who had regular follow-up and who had been recorded in the medical records of Çukurova University, Faculty of Medicine, Balcali Hospital were contacted by phone and scheduled for a face-to-face interview. For five patients who died during the postoperative period, mandatory information was collected through family members or from retrospective assessment of their medical records. Each participant had pathologically verified bronchiectasis.
This study consisted of two-phases: face-to-face interview and retrospective analysis of medical records. Sociodemographic characteristics (age, gender, occupation, and comorbidities) and changes in respiratory symptoms compared to the preoperative period were evaluated for each participant who was interviewed face to face after receiving informed consent. Retrospective data were gathered by using questions posed during face-to-face interviews as well as records from hospitals and health systems.
Preoperative evaluation
Each participant’s preoperative examination included complete sociodemographic data and symptom analysis, as well as imaging modalities (high resolution computed tomography) to determine the number of affected lobes and a full pulmonary function test.
Pulmonary function tests (PFTs) were performed by using a calibrated Sensor Medics V-Max 20 Spirometer (Jaeger MS-PFT Analyser Unit, Wiasys Healthcare GmbH, Höchberg, Germany). The patients had not received any oral or inhaled short-acting beta-2 agonists within 8 hours of testing. Baseline forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) were measured three times, and the best of the three measurements was recorded. Total lung capacity was measured using the helium dilution technique (Jaeger MS-PFT Analyser Unit, Wiasys Healthcare GmbH). The diffusing capacity of the lung for carbon monoxide (DLCO) was measured by using the single breath method. The results are presented as the percentages of predicted values.
Surgical procedures
Each surgery was performed after single-lung ventilation was established through a double-lumen endotracheal tube. A thoracotomy was performed on 87 patients, and 15 patients were treated by video-assisted thoracoscopic surgery.
Postoperative treatment and follow-up
Following the procedure, each participant in the trial was visited daily, and appropriate diagnostic tests and management of early postoperative complications were applied.
Definitions of postoperative complications
The European perioperative clinical outcome definitions were used in the diagnosis of postoperative complications [14]. Early postoperative complications are defined as those observed until discharge and late postoperative complications are defined as those detected during follow-up visits.
Statistical Analysis
SPSS 22 program (IBM Corp., Armonk, NY, USA) was used in the analysis of the data. Data are presented as number, percentage, arithmetic mean, standard deviation, median, interquartile range. Kolmogorov Smirnov test was used as the normal distribution test. Parametric tests were preferred in the analysis of normally distributed data, and non-parametric tests were preferred in the analysis of non-normally distributed and categorical data. Mann Whitney U test, t test, Chi-square test, Binary logistic regression test, Cox regression test were used in the analysis. Binary logistic regression analysis was used to estimate the risk of developing complications and Cox regression analysis was used to estimate the mortality risk. A value of p < 0.05 was considered statistically significant.
Results
The 102 patients included 47 (46.1%) males and 55 (53.9%) females, with an average age of 38.8 ± 14.0 years. Prior to surgery, all patients had had a productive cough for 12.3 ± 3.2 months.The indication for surgery was hemoptysis in 16 (15.7%), recurrent pulmonary infections in 74 (72.5%), and suspicion of malignant transformation in 12 (11.8%) of the patients. The mean follow-up period was 71.4 ± 28 months (median: 66 months; min: 0, max: 119 months). The average sputum quantity expectorated per day was 85.6 ± 57 mL. Of note, 76 (74.2%) patients had had at least one hemoptysis episode in their life.
A total of 116 surgical procedures were applied to the 102 patients. Bilateral surgery was performed in two patients. The surgery was thoracotomy in 87 (85.3%) patients and video-assisted thoracoscopic surgery in 15 (14.7%) patients. Specifically, 10 (9.8%) of the surgeries were pneumonectomy, 67 (65.7%) were lobectomy, 12 (11.8%) were bilobectomy, 23 (22.5%) were segmentectomy, and 4 (3.9%) had wedge resection. The most common surgery was left lower lobectomy (n = 30, 29.4%). The most common type of segmentectomy was lingulectomy (n = 14, 13.7%).
There was no intraoperative mortality. Perioperative complications were seen in 22 (21.5%) patients. One patient died due to respiratory failure during the early postoperative period (postoperative day 3). Five (4.9%) patients died during long-term follow-up. Sociodemographic characteristics and perioperative results of the patients are given in table 1.
Table 1: Sociodemographic and disease-related characteristics.
When examining the frequencies of complications, long-term ex status, and improvement in symptoms were examined according to the surgical procedure utilized (laparoscopic/thoracotomy), there were no significant differences (Table 2).
Table 2: Postoperative complications according to surgery technique.
The logistic regression model established to predict the development of complications was significant (Omnibus test p = 0.016). The dependent variable of the model is the presence of complications and the independent variables are the technique used in the treatment (reference category: thoracotomy) and the type of bronchiectasis (reference category: cylindrical type). Among the variables included in the model, the risk of developing complications in the presence of varicose bronchiectasis, in which the type of bronchiectasis is important, was 8.44 times higher than in cylindrical bronchiectasis (Table 3).
Table 3: Estimation of postoperative complications in Logistic Regression Analysis.
The preoperative PFTs were obstructive in 33 (32.4%), restrictive in 21 (20.6%), and normal in 48 (47.1%) patients. When comparing the PFT values according to the postoperative complication, the FVC and DLCO values were significantly lower in the patients who had developed complications (Table 4).
Table 4: Comparison of PFTs according to postoperative complications.
In the Cox regression analysis performed to evaluate the factors affecting long-term survival, the technique used was not important for survival, but the development of complications was important. Specifically, the risk of mortality in the later period was 13.92 times higher in patients who had developed complications compared with patients who had not developed complications (Table 5, Figure 1).
Table 5: Cox Regression Analysis for estimation of mortality.
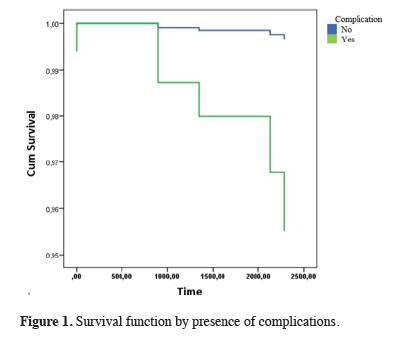 Click Here to Zoom |
Figure 1: Survival function by presence of complications. |
Discussion
Early postoperative complications after non-cystic fibrosis bronchiectasis surgery were found in 21.5% of our patients, with no intraoperative fatality and 4.9% mortality in the long-term follow-up. The most common postoperative complications were surgical site infection and hemorrhage. The type of bronchiectasis (varicose bronchiectasis) and lower FVC and DLCO values in the perioperative period were identified as risk factors for the development of postoperative complications. Long-term mortality was also higher in individuals who had had early postoperative complications.Surgical site infection and hemorrhage were the most prevalent early postoperative complications. A previous study comparing surgical and conservative treatment strategies in bronchiectasis revealed an early postoperative complication rate as 33.5%; the complications were mild in 64% of the cases [15]. An older study with a high mortality rate showed 27.4% of the patients had postoperative complications [16]. In another study dealing with unilateral bronchiectasis cases, there were postoperative complications in 15% of patients; the most common was bleeding [13]. Operative morbidity was seen in 11% of patients, with 3.5% experiencing serious and 7.5% experiencing mild complications [17]. A Portuguese study concluded that the postoperative complication rate including temporary broncho-pleural fistulae, hemorrhage, and atrial arrhythmias was 15% [18]. In a study with an average hospitalization period of 8.5 days, the morbidity rate was 16%, including prolonged air leaks, expansion defects, and wound infections [19]. A more recent study determined that the incidence of postoperative complications was 14.6% [20]. A comprehensive study revealed postoperative complications in 19.6% of the participants; these complications included sputum retention, postoperative air leak for more than 2 weeks, empyema, pneumonia, bronchopleural, atelectasis, postoperative bleeding, and respiratory insufficiency that required mechanical ventilation [21]. A recent study in Europe detected complications including atelectasis (15%), pleural effusion (21%), or prolonged air leak (21%) in 53% of the patients with bronchiectasis [22]. In a study dealing with surgery of a destroyed lung lobe, the postoperative mortality rate was 10.6% [23]. In a recent study with a large number of patients, postoperative complications (most commonly wound infections, atelectasis, and prolonged air leak) were observed in 9.7% of patients [24]. A large study from China reported a postoperative complication rate of 16.2% [25]. The data from the present study are compatible with the literature.
This study revealed no intraoperative fatality and a 4.9% mortality rate in the long-term follow-up. A very old study showed an early postoperative mortality rate of 3.1% and an overall postoperative mortality rate of 3.5%, possibly due to underdeveloped surgical techniques [16]. As time has passed, new studies have revealed better results of surgery in bronchiectasis. In a small study, patients with unilateral bronchiectasis had no intraoperative death and no late deaths [13]. In 1982, Annest et al [26] reported late deaths in 8% of patients, attributable to pulmonary causes. Three studies from Turkey showed a 1%-1.7% operative mortality rate in surgical treatment of bronchiectasis [17,19,20]. In a study conducted in a series of 790 patients, there was no intraoperative mortality, while the postoperative mortality rate was 1.1%. The reasons of death were mainly pneumonia, pulmonary embolism, and acute respiratory distress syndrome [25]. A study of 90 patients from Germany showed no operative mortality in the first 30 days after surgery [21]. A recent study revealed that 3.3% of the participants had died due to postoperative complications, namely due to acute myocardial infarction [22]. Another recent study showed a 90-day mortality rate of 0% [24]. Both intraoperative and postoperative mortality in the present study are within tolerable limits and are consistent with the literature. Based on these findings, it can be concluded that surgery can be a preferred therapeutic option for non-cystic fibrosis bronchiectasis under certain circumstances.
In the present study, varicose bronchiectasis and lower FVC and DLCO values in the perioperative period were identified as risk factors for the development of postoperative complications, and long-term mortality was higher in individuals who had developed early postoperative complications. A study evaluating risk factors of postoperative results showed that complete resection independently predicted a symptom-free outcome, an FEV1 of less than 60% of the predicted value predicted an incomplete resection, and preoperative antibiotic therapy independently predicted postoperative complications [20]. Another study showed that complete recovery was rarely achieved in patients with Pseudomonas aeruginosa infection and obstructive airway disease, but the number of patients was very small. Moreover, neither FEV 1 nor FVC alone was of use for predicting the outcome of surgery while the FEV1/FVC ratio was significant in prediction of cure [13]. In a previous study, logistic regression extracted the type of bronchiectasis (cylindrical), the existence of sinusitis, and the type of resection (complete/incomplete) for prognostic discrimination with statistical significance [21]. A large study from China showed that only a preoperative FEV1 of < 50% was associated with postoperative complications in univariate analyses. Multivariate analysis found no factors that were significantly associated with postoperative complications. The same study revealed that preoperative renal failure was associated with an elevated risk of postoperative death. The logistic regression analysis of a large study showed that tuberculous bronchiectasis, the type of bronchiectasis (saccular versus others), and the type of resection (incomplete or complete) were three independent factors associated with a poor surgical outcome [25]. There have only been a few studies about surgery in non-cystic fibrosis bronchiectasis. Of course, it is challenging to identify risk factors with such limited data. Nevertheless, researchers have identified various risk factors, and our findings have added to the literature in this regard.
In the present study, nearly three quarters of the patients with non-cystic fibrosis bronchiectasis who underwent surgical resection had improved symptoms. An older study showed 71% improved symptoms after surgical intervention of bronchiectasis [16]. In a Turkish study, after surgical treatment, 82.5% of patients were free of symptoms and the remaining 17.5% had a reduction in preoperative symptoms [20]. In a study conducted with a limited number of patients and in which patients with unilateral bronchiectasis were evaluated, 72.5% reported complete recovery, while 27.2% showed improvement in symptoms [13]. In another study from Turkey, the pulmonary symptoms disappeared in 66.9% of the patients after surgery, and only 3.6% of patients had persistent or worsened symptoms [17]. A recent study showed 67% of patients considered their own health as “excellent” after surgery, 30% as “good,” and 3% reported “no change” [22]. A detailed study showed that 60.5% of the patients were asymptomatic, 14.1% had improved symptoms, and 14.8% showed no improvement or worsened conditions [25]. The data from the present study is consistent with the literature and reinforces the effectiveness of surgery on symptom relief.
Although the study was done in Turkey, one of the nations where tuberculosis is rampant and bronchiectasis is relatively common, the study’s main limitation is the small number of patients from a single center. Because the research was carried out at a tertiary health care institution, the individuals admitted are frequently advanced cases who have had trouble receiving treatment at other health centers. This might explain why the postoperative morbidity was somewhat greater than predicted.
Despite breakthroughs in preventative measures, medical therapy, and follow-up, surgery, with its low mortality and morbidity rates, remains a viable option for the treatment of non-cystic fibrosis bronchiectasis in selected individuals. This option meets the patients’ clinical and social expectations.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Approval was granted by the Ethics Committee of Cukurova University (Protocol No: 122/2022).
Authors’ contribution
OBT,AA,EG; material preparation, data collection and analysis, OBT,BM, EG; the first draft of the manuscript was written by, All authors read and approved the final manuscript. All authors contributed to the study conception and design.
Reference
1) Cole PJ. Inflammation: a two-edged sword-the model of bronchiectasis. Eur J Respir Dis 1986; 147: 6-15.
2) Fan LC, Liang S, Lu HW, Fei K, Xu JF. Efficiency and safety of surgical intervention to patients with Non-Cystic Fibrosis bronchiectasis: a meta-analysis. Sci Rep 2015; 5: 17382.
3) Hiramatsu M, Shiraishi Y, Nakajima Y, Miyaoka E, Katsuragi N, Kita H et al. Risk factors that affect the surgical outcome in the management of focal bronchiectasis in a developed country. Ann Thorac Surg 2012; 93: 245-50
4) Ringshausen FC, de Roux A, Pletz MW, Hämäläinen N, Welte T, Rademacher J. Bronchiectasis-associated hospitalizations in Germany, 2005-2011: a population-based study of disease burden and trends. PLoS One 2013; 8: e71109.
5) Weycker D, Edelsberg J, jOster G, Tino G. Prevalence and economic burden of bronchiectasis. Clin Pulm Med 2005; 12: 205-9.
6) Kwak HJ, Moon JY, Choi YW, Kim TH, Sohn JW, Yoon HJ et al. High prevalence of bronchiectasis in adults: analysis of CT findings in a health screening program. Tohoku J Exp Med 2010; 222: 237-42.
7) Quint JK, Millett ER, Joshi M, Navaratnam V, Thomas SL, Hurst JR et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016; 47: 186-93.
8) Chang AB, Grimwood K, Mulholland EK, Torzillo PJ; Working Group on Indigenous Paediatric Respiratory Health. Bronchiectasis in indigenous children in remote Australian communities. Med J Aust 2002; 177: 200-4.
9) Seitz AE, Olivier KN, Steiner CA, Montes de Oca R, Holland SM, Prevots DR. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993-2006. Chest 2010; 138: 944-9.
10) Finklea JD, Khan G, Thomas S, Song J, Myers D, Arroliga AC. Predictors of mortality in hospitalized patients with acute exacerbation of bronchiectasis. Respir Med 2010; 104: 816-21.
11) Goeminne PC, Nawrot TS, Ruttens D, Seys S, Dupont LJ. Mortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysis. Respir Med 2014; 108: 287-96.
12) Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629.
13) Ashour M, Al-Kattan KM, Jain SK, Al-Majed S, Al-Kassimi F, Mobaireek A et al. Surgery for unilateral bronchiectasis: results and prognostic factors. Tuber Lung Dis 1996; 77: 168-72.
14) Jammer I, Wickboldt N, Sander M, Smith A, Schultz MJ, Pelosi P et al; European Society of Anaesthesiology (ESA) and the European Society of Intensive Care Medicine (ESICM); European Society of Anaesthesiology; European Society of Intensive Care Medicine. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015; 32: 88-105.
15) Sanderson JM, Kennedy MC, Johnson MF, Manley DC. Bronchiectasis: results of surgical and conservative management. A review of 393 cases. Thorax 1974; 29: 407-16.
16) Doğan R, Alp M, Kaya S, Ayrancioğlu K, Taştepe I, Unlü M et al. Surgical treatment of bronchiectasis: a collective review of 487 cases. Thorac Cardiovasc Surg 1989; 37: 183-6.
17) Kutlay H, Cangir AK, Enön S, Sahin E, Akal M, Güngör A et al. Surgical treatment in bronchiectasis: analysis of 166 patients. Eur J Cardiothorac Surg 2002; 21: 634-7.
18) Prieto D, Bernardo J, Matos MJ, Eugénio L, Antunes M. Surgery for bronchiectasis. Eur J Cardiothorac Surg 2001; 20: 19-23, discussion 23-4.
19) Gursoy S, Ozturk AA, Ucvet A, Erbaycu AE. Surgical management of bronchiectasis: the indications and outcomes. Surg Today 2010; 40: 26-30.
20) Balci AE, Balci TA, Ozyurtan MO. Current surgical therapy for bronchiectasis: surgical results and predictive factors in 86 patients. Ann Thorac Surg 2014; 97: 211-7
21) Fujimoto T, Hillejan L, Stamatis G. Current strategy for surgical management of bronchiectasis. Ann Thorac Surg 2001; 72: 1711-5.
22) Selman A, Merhej H, Nakagiri T, Zinne N, Goecke T, Haverich A et al. Surgical treatment of non-cystic fibrosis bronchiectasis in Central Europe. J Thorac Dis 2021; 13: 5843-50.
23) Kabiri EH, Hammoumi ME, Bhairis M, Oueriachi FE, Slaoui O, Amraoui M. Clinical and surgical analysis of lobectomy for destroyed lobe of the lung: A series of 47 patients. Asian Cardiovasc Thorac Ann 2021; 29: 772-8.
24) Gülhan SŞE, Acar LN, Sayılır Güven E, Bıçakçıoğlu P, Aydın E, Karasu S et al. Surgical treatment of bronchiectasis: Our 23 years of experience. Turk Gogus Kalp Damar Cerrahisi Derg 2020; 28: 629-37.



