

Summary
Background: The aim of this study is to examine the recurrence rates and their causes after axillary thoracotomy and VATS for the treatment of primary spontaneous pneumothorax (PSP).Materials and Methods: Patients who were operated due to PSP in our department between January 2011 and December 2016 were analysed retrospectively. Differences in age, sex, operation side, number of chest tubes inserted during operation, duration of postoperative drainage, need for Heimlich valve system, smoking habit and recurrence were examined between two groups of patients, who underwent axillary thoracotomy and VATS.
Results: Among 180 patients operated during this period, 98 (54.4%) patients underwent VATS, and 82 (45.6%) patients underwent axillary thoracotomy. The recurrence rate was 6.1% (n=6) in VATS patients, while it was 8.5% (n=7) after axillary thoracotomy, and the difference was not statistically significant (p=0.533). Eight of 13 patients with recurrences were found to be active smokers in the postoperative period. It was found that active smoking increased the incidence of recurrences from 3.6% to 19%, and the difference was statistically significant (p=0.001).
Conclusions: We conclude that patients diagnosed with PSP can be operated by videothoracoscopy since there was no significant difference in the recurrence rates after VATS and axillary thoracotomy, and VATS is a more minimally invasive surgical intervention and causes less incision scar compared to axillary thoracotomy. Because of the significant increase in the postoperative recurrence rates, smoking cessation should be recommended to the patients with PSP and necessary precautions should be taken for this purpose.
Introduction
Pneumothorax can be defined as air trapping between the parietal and visceral pleura. It may occur spontaneously as well as due to blunt and penetrating thoracic trauma. It is called secondary spontaneous pneumothorax when it is observed after a parenchymal disease, especially tuberculosis and emphysema. However, if there is no underlying disease, it is called primary spontaneous pneumothorax (PSP).The biggest problem of spontaneous pneumothorax is the high recurrence rate of the disease [1-4]. Surgical approach is the most effective and reliable treatment method to reduce the recurrence rate. When recurrence is detected, PSP must be surgically treated in the first attack in the patients with synchronous or metachronous bilateral pneumothorax, with prolonged air leak (PAL), and who have a high-risk occupation [5-7]. For a long time, thoracotomy with blebectomy (apical wedge resection) and apical pleurectomy had been performed as a surgical procedure, and axillary thoracotomy had been preferred on account of less muscle incision and better cosmetic results. Video-assisted thoracic surgery (VATS) has been used more frequently in patients operated due to PSP after the introduction of videothoracoscopic procedures.
In our study, we compared recurrence rates and causes after axillary thoracotomy and VATS in the patients with PSP.
Methods
Patients who were operated due to PSP in our department between January 2011 and December 2016 were analysed retrospectively. The pieces of information as to smoking history, occupation, ordinal number of the recurrence, pneumothorax side, and cause of operation were taken from the medical recordings of the patients operated. In operation indications, ipsilateral recurrent pneumothorax was classified as "recurrent pneumothorax", contralateral pneumothorax as "contralateral recurrent pneumothorax", and air leak that lasts more than 7 days as "prolonged air leak (PAL)".Routine blood tests were performed preoperatively in all patients and posteroanterior chest radiographs were taken. Thoracic computed tomography (CT) was used for parenchymal evaluation.
All patients underwent double lumen intubation under general anaesthesia regardless of the method. Thoracotomy was performed using approximately 10 cm of axillary thoracotomy incision with the standard method of muscle preservation, and VATS was performed using 2 ports. Wedge resection and apical pleurectomy were performed on the patients regardless of the method, and the operations were terminated with the insertion of 1 or 2 chest tubes depending on the surgeon"s decision.
In the postoperative period, follow-up data of the patients were obtained from the hospital records and from the patients by phone. The relationship between postoperative recurrence and age (patients were divided into two groups as under the age of 25 and aged 25 and above), sex, type of surgical approach, operation side, number of chest tubes inserted during operation, duration of postoperative drainage (two groups as 5 days and less, and longer than 5 days), need for Heimlich valve system, and smoking habit was studied.
Statistical Analysis
The SPSS 24 package program was used to analyse recurrence rates and other related variables. The comparative statistics were based on the "Pearson Chi-Square" test. A p value < 0.05 was considered statistically significant.
Results
It was observed that 180 patients had been operated with the diagnosis of PSP in our department between the aforementioned dates. 164 patients (91.1%) were male and 16 (8.9%) were female. The mean age was 25.1±7.6; the youngest patient was 14, and the oldest patient was 49. 102 (56.7%) patients were under 25 and 78 (43.3%) patients were at the age of 25 and older. The majority of the patients were students because of the frequent occurrence in young patients.Among 180 patients in total, 122 patients (67.8%) were operated due to the same side recurrence and 52 (28.9%) patients were operated due to PAL in the first attack. Only 6 (3.3%) of the patients operated had contralateral recurrent pneumothorax. We did not have a patient who had a high-risk occupation and was therefore operated in the first attack. 95 (52.8%) patients were operated on the right side, and 85 (47.2%) patients were operated on the left side (Table 1).
Table 1: The results and comparison of axillary thoracotomy and VATS.
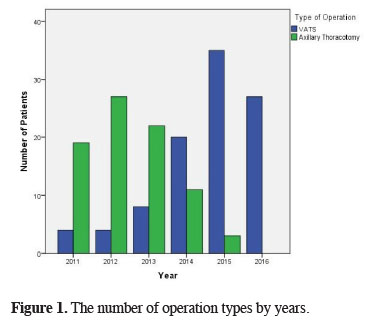
VATS was performed on 98 (54.4%) patients, whereas 82 (45.6%) patients underwent axillary thoracotomy. It was seen that VATS is more preferred especially in recent years (Figure 1). It was also observed that apical wedge resection (bullectomy and/or blebectomy) and apical pleurectomy were performed on all of the patients independently of the method. The mean length of follow-up was 35.5 ± 20.3 months. The follow-up duration was 50.4 ± 13.5 months in patients undergoing thoracotomy and 22.9 ± 16.1 months in patients undergoing VATS.
 Click Here to Zoom |
Figure 1: The number of operation types by years. |
At the end of the operation, two chest tubes were inserted into 121 (67.2%) patients, and single chest tube was inserted into 59 (32.8%) patients. The total duration of drainage was 5 days and less in 107 (59.4%) patients and longer than 5 days in 73 (40.6%) patients, independently of the number of chest tubes. A total of 18 (10%) patients required long-term drainage using "Heimlich Valve". It was found out that 42 (23.3%) patients kept smoking in the postoperative period as well. In total, 13 (7.2%) patients had recurrence in the postoperative period.
There was no significant difference between the groups of VATS and axillary thoracotomy in terms of sex (p = 0.154), age (p = 0.456), operation indication (p = 0.066), operation side (p = 0.2), number of chest tubes (p = 0.549), duration of drainage (p = 0.595), application of Heimlich valve system (p = 0.549) and active smoking (p = 0.069). While the recurrence rate was 6.1% (n = 6) in the patients operated by VATS, it was 8.5% (n = 7) after axillary thoracotomy, and the difference was not statistically significant (p = 0.533) (Table 1).
There was no significant difference between recurrence rates and sex (p = 0.242), age (p = 0.343), operation side (p = 0.511), operation indication (p = 0.669), number of chest tubes (p = 0.873), duration of drainage (p = 0.183), and application of Heimlich valve system (p = 0.212), regardless of the method.
Eight of 13 patients who had recurrence were found to be active smokers in the postoperative period. It was found out that active smoking increased the incidence of recurrence from 3.6% to 19%, and the difference was statistically significant (p = 0.001) (Table 2).
Table 2: The analysis of factors effecting recurrence after both surgical procedures.
After recurrence, 3 (23.1%) patients were followed by oxygen inhalation, thoracentesis was applied to 1 (7.7%) patient, and 8 patients (61.5%) underwent tube thoracostomy; however, only one (7.7%) of those patients had reoperation by VATS.
Discussion
The most important problem of PSP is the high recurrence rate of the disease [1-4]. Surgical approach is the most effective and reliable treatment method to reduce the recurrence rate. It is stated that patients with synchronous or metachronous bilateral pneumothorax, with prolonged air leak (PAL), and who have a high-risk occupation must be operated when recurrence is detected [5-7]. It is reported in some of the studies that patients can be surgically treated by VATS in the first attack [3,8,9]. In our department, only the patients with PAL underwent surgical treatment in the first attack. Despite the fact that Treasure et al. considered 4 or 5 days as a criterion for PAL, patients who had had PAL for 7 days or longer were included in the PAL group in our department [4].During the operation, it is aimed to eliminate the air leak, to resect the bullae and blebs completely, and to provide pleural adhesion so as to prevent recurrences. Wedge resection and apical pleurectomy were hence performed on all patients independently of the method.
Although Zhang et al. stated that pleural abrasion had no significant recurrence effect compared to bullectomy in the patients for whom propensity score matching was implemented, in the same study the recurrence rate was 2.6% in the group undergoing pleurectomy and 5.3% in the group which pleurectomy was not performed on. [10]. According to the results of the studies revealing a high recurrence rate of 16.1 to 16.3% in the patients undergoing bullectomy and the fact that pleurectomy was superior to abrasion and that this could be confined to apical region, there were no patients undergoing only mechanical abrasion in our department [7,11,12]. There are some publications which mention about the fact that postoperative haemorrhage is observed more frequently in apical pleurectomy compared to the other methods, and about the need for reoperation due to haematoma; however, there are no patients who had undergone a reoperation on account of haematoma in our series [13,14].
In our department, thoracotomy with blebectomy (apical wedge resection) and apical pleurectomy had been performed as a surgical procedure for a long period of time. The axillary thoracotomy had been preferred on account of less muscle incision and for better cosmetic results. After the widespread use of the videothoracoscopic applications, VATS has been more frequently used in patients operated due to PSP. In parallel with the worldwide trend, the applications of VATS in the surgical treatment of PSP have almost eliminated the axillary thoracotomy in our department.
The recurrence rates are variable in studies among patients operated by VATS and axillary thoracotomy. Inderbitzi et al. found the recurrence rate 8.3% in 163 patients who underwent VATS in their prospective study [15]. Recurrence was detected after VATS in 4% of the patients by Bertrant et al., in 0.4 to 8.6% of the patients by Divisi et al., and in 11.4% of the patients by Chang et al. [3,16,17]. There are studies reporting that recurrence rates are higher in patients operated by VATS [11,18-21]. Although higher recurrence rates in VATS is based on the differences in pleurectomy techniques in the literature [19,22], Sedrakyan et al. reported that the recurrence rate after VATS was significantly lower in the axillary thoracotomy [23]. In our study, the recurrence rate was 6.1% in VATS patients and 8.5% in axillary thoracotomy patients, and the difference was not statistically significant (p = 0.533). Although the recurrence rate after axillary thoracotomy is not statistically significant, the fact that it is higher than VATS may be considered as a result of the selection of cases that are deemed to be relatively more complicated.
Although some studies indicates that there is no significant relation between smoking and postoperative recurrence rates, most studies report that recurrence rates increase significantly [9,13,17,24]. As also noted in the British Thoracic Society"s guideline with the topic of "Management of Spontaneous Pneumothorax", smoking raises the possibility of PSP in healthy individuals up to 12% [5]. It is also reported that the possibility of recurrence increases up to 54% in the PSP patients who kept smoking after the first attack [5]. In our study, regardless of the method, the recurrence rate was 19% in the patients who actively smoked cigarettes in the postoperative period, while it was only 3.6% in the group of non- and ex-smokers. The difference was statistically significant (p = 0.001).
As a conclusion patients diagnosed with PSP can be operated by videothoracoscopy since there was no significant difference in the recurrence rates after VATS and axillary thoracotomy, and VATS is a more minimally invasive surgical intervention and causes less incision scar compared to axillary thoracotomy. In the light of the results, because of the significant increase in the postoperative recurrence rates, smoking cessation should be recommended to the patients receiving treatment due to PSP, and necessary precautions should be taken for this purpose.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Reference
1) Tulay CM, Özsoy İE. Spontaneous pneumothorax recurrence and surgery. Indian J Surg 2015; 77: 463-5.
2) Chen YY, Huang HK, Chang H, Lee SC, Huang TW. Postoperative predictors of ipsilateral and contralateral recurrence in patients with primary spontaneous pneumothorax. J Thorac Dis 2016; 8: 3217-24.
3) Divisi D, Di Leonardo G, Crisci R. Video-assisted thoracic surgery versus pleural drainage in the management of the first episode of primary spontaneous pneumothorax. Am J Surg 2015; 210: 68-73.
4) Treasure T, Hallifax RJ, Rahman N. Are we ready to go directly to videothoracoscopic surgery at a first presentation of primary spontaneous pneumothorax? Eur J Cardiothorac Surg 2016; 49: 860-1.
5) MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65: 18-31.
6) Fry WA, Paape K. Pneumothorax. In: Shields TW, editor. General Thoracic Surgery. 6th ed. Philadelphia: Willams and Wilkins; 2005. p. 794-805.
7) Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001; 119: 590-602.
8) Herrmann D, Klapdor B, Ewig S, Hecker E. Initial management of primary spontaneous pneumothorax with video-assisted thoracoscopic surgery: a 10-year experience. Eur J of Cardiothorac Surg 2015; 49: 854-9.
9) Al-Mourgi M, Alshehri F. Video-Assisted Thoracoscopic Surgery for the treatment of first-time spontaneous pneumothorax versus conservative treatment. Int J Health Sci 2015; 9: 428-32.
10) Zhang Z, Du L, Feng H, Liang C, Liu D. Pleural abrasion should not routinely preferred in treatment of primary spontaneous pneumothorax. J Thorac Dis 2017; 9: 1119-25.
11) Takahashi R. Evaluation of spontaneous pneumothorax surgeries: a 16-year experience in Japan. Surg Res Prac 2016.
12) Jeon HW, Kim YD, Kye YK, Kim KS. Air leakage on the postoperative day: powerful factor of postoperative recurrence after thoracoscopic bullectomy. J Thorac Dis 2016; 8: 93-7.
13) Kocatürk Cİ, Cansever L, Günlüoğlu MZ, Turna A, Özdemir S, Çınar U, et al. The results of wedge resection and partial pleurectomy in the surgical treatment of primary spontaneous pneumothorax: videothoracoscopy or axillary thoracotomy? Turkish J Thorac Cardiovasc Surg 2011; 19: 213-7.
14) Gossot D, Galetta D, Stern JB, Debrosse D, Caliandro R, Girard P, et al. Results of thoracoscopic pleural abrasion for primary spontaneous pneumothorax. Surg Endosc 2004; 18: 466-71.
15) Inderbitzi RG, Leiser A, Furrer M, Althaus U. Three years" experience in video-assisted in thoracic surgery (VATS) for spontaneous pneumothorax. J Thorac and Cardiovasc Surg 1994; 107: 1410-5.
16) Bertrand PC, Regnard JF, Spaggiari L, Levi JF, Magdeleinat P, Guibert L, et al. Immediate and long-term results after surgical treatment of primary spontaneous pneumothorax by VATS. Ann Thorac Surg 1996; 61: 1641-5.
17) Chang JM, Lai WW, Yen YT, Tseng YL, Chen YY, Wu MH, et al. Apex-to-cupola distance following VATS predicts recurrence in patients with primary spontaneous pneumothorax. Medicine (Baltimore) 2015; 94: e1509.
18) Freixinet JL, Canalís E, Juliá G, Rodriguez P, Santana N, de Castro FR. Axillary thoracotomy versus videothoracoscopy for the treatment of primary spontaneous pneumothorax. Ann Thorac Surg 2004; 78: 417-20.
19) Kim KH, Kim HK, Han JY, Kim JT, Won YS, Choi SS. Transaxillary minithoracotomy versus video-assisted thoracic surgery for spontaneous pneumothorax. Ann Thorac Surg 1996; 61: 1510-2.
20) Barker A, Maratos EC, Edmonds L, Lim E. Recurrence rates of video-assisted thoracoscopic versus open surgery in the prevention of recurrent pneumothoraces: a systematic review of randomised and non-randomised trials. The Lancet 2007; 370: 329-35.
21) Pagès PB, Delpy JP, Falcoz PE, Thomas PA, Filaire M, Le Pimpec Barthes F, et al. Videothoracoscopy versus thoracotomy for the treatment of spontaneous pneumothorax: a propensity score analysis. Ann Thorac Surg 2015; 99: 258-63.
22) Rena O, Massera F, Papalia E, Della Pona C, Robustellini M, Casadio C. Surgical pleurodesis for Vanderschueren"s stage III primary spontaneous pneumothorax. Eur Respir J 2008; 31: 837-41.



