

2Department of Chest Diseases, University of Health Sciences, Dr. Suat Seren Chest Diseases and Surgery Medical Practice and Research Center, İzmir, Turkey DOI : 10.26663/cts.2019.00020
Summary
Background: This study aims to evaluate the mortality and survival rates of patients undergoing pneumonectomy due to primary non-small cell lung cancer (NSCLC) and to identify the factors which affect these variables.Materials and Methods: This retrospective single center cohort study included a total of 250 patients who underwent pneumonectomy due to NSCLC between January 2007 and July 2015. 30- and 90-day mortality rates and survival rates of the patients were analyzed. The factors which affected survival and mortality were evaluated.
Results: The mean age was 59 (range: 35 to 77) years. The median survival was 48.1 months (95% C. I. 30.6 – 65.6 months) and five-year survival rate was 45.6%. The 30- and 90-day mortality rates were 6.4% and 7.6%, respectively. Age, nodal disease, and complete resection were factors to affect the survival (p = 0.002, p = 0.0001 and p = 0.017, respectively). Age was also effective on 30- and 90-day mortality (p = 0.004, RR: 3.3 and p = 0.006, RR: 2.5, respectively). The early rethoracotomy rate was 5.6% (n = 14) and the postoperative mortality rate in these patients was 28.6% (n = 4).
Conclusions: Although implementation of pneumonectomy due to lung cancer seems fearful, the present study found lower survival rates only depending on advanced age and N2 disease. The high levels of 30- and 90-day mortality and the increased mortality after early rethoracotomy was associated with advanced age and comorbidity. In terms of postoperative mortality, the selection of eligible patients is important in elderly patients who are candidates of pneumonectomy due to lung cancer.
Introduction
Prior to 1930, pneumonectomy was fatal due to bleeding, the lack of durable bronchial closure methods and antisepsis. Later, lobectomy were shown to have similar efficacy along with less morbidity, leading to gradually reduced indications for pneumonectomy [1]. However, pneumonectomy may be still inevitable due to anatomical and technical reasons. As it is associated with higher morbidity and mortality, compared to other lung resections, the effect of pneumonectomy on long-term survival is still controversial.In the present study, it was aimed to evaluate the mortality and survival rates of patients who underwent pneumonectomy due to primary non-small cell lung cancer (NSCLC) and to identify the factors affecting these variables.
Methods
A written informed consent was obtained from each patient. The study was approved by the institutional study board and was conducted in accordance with the principles of the Declaration of Helsinki.This retrospective study included a total of 250 patients who hable ad pneumonectomy due to NSCLC between January 2007 and July 2015. Patients who had completion pneumonectomy, pneumonectomy due to an indication other than NSCLC were excluded. Data including age, histology, lymph node metastasis, T status, neoadjuvant therapy, adjuvant therapy, and the state of complete and incomplete resection at the surgical margin during post-resection histopathological examinations were obtained from hospital records operation reports, patient charts, and national survival database. All patients were analyzed in terms of 30- and 90-day mortality rates and survival. The survival time was calculated as the duration between the days of operation and death. The factors affecting survival and mortality were assessed statistically. Cardiac disease, hypertension, diabetes mellitus and previous tuberculosis were recorded as comorbidities.
Preoperative assessment included posteroanterior chest x-ray, thoracic, and upper abdominal computed tomography (CT), bronchoscopy, positron emission tomography, cranial magnetic resonance imaging or CT, pulmonary function tests (PFT) and blood gas analysis as a standard procedure. For eligible patients, transthoracic fine-needle aspiration biopsy, and mediastinal lymph node staging with endobronchial ultrasonography and/or mediastinoscopy were performed. The patients with the forced expiratory volume in 1 second (FEV1) level >2 l or 70% as measured by PFT underwent pneumonectomy prior to any advanced respiratory assessment. For the patients with PFT levels considered insufficient; quantitative perfusion scintigraphy, maximal oxygen consumption (VO2peak) and stair climbing tests were performed. Respiratory physiotherapy was applied as guided by physiotherapists in the postoperative period. Smoking patients were ensured to quit smoking at least one week before the operation.
Prior to thoracotomy, all patients were administered antibiotic and pulmonary embolism prophylaxis. During the operation, anesthesiologists conducted double-lumen intubation, arterial and central venous pressure monitorization and epidural analgesia as a standard procedure. The serratus anterior muscle was preserved and posterolateral thoracotomy was performed. To achieve a complete resection, additional procedures such as intrapericardial procedures or chest wall resection as well as extended pneumonectomy were performed when necessary. For bronchial closure, mostly stapler was used upon surgeon’s choice. Each patient underwent systematic mediastinal and hilar lymph node dissection. The surgical margins were assessed by a pathologist using the frozen-section procedure. At the end of the operation, a thoracic drain was inserted. Extubation was ensured in the operating room as far as possible and the patients were kept under supervision for 24 to 48 hours in the intensive care unit. The amount of fluid drainage was followed and the thoracic drain was removed within the first 72 hours. The discharged patients were followed in the outpatient setting. All complications and mortalities were recorded.
Statistical Analysis
Data were collected from hospital database, operational reports, patient charts and national mortality database. Excel software (Microsoft Corp, Seattle, WA) was used to analyze the data. The means and standard deviations of the continuous variables, and number and percent of categorical variables were given by using descriptive statistics. Statistical analysis was performed using the SPSS 16.0 software program (SPSS Inc., Chicago, IL, USA). All cumulative survival curves were estimated using the Kaplan–Meier method, and differences between the groups were evaluated by the log-rank test. The overall survival (OS) was considered as the time from the date of initial surgery to the date of death from any cause or the date of the last follow-up. Variables with a P-value less than 0.1 were selected for further multivariable analysis. Multivariable analysis for OS was performed to identify the prognostic factors using the Cox proportional hazard model.
Results
During the study period, there were a total of 1,267 patients who had lobectomy and pneumonectomy due to primary NSCLC in our clinic, and 1,017 (80.3%) of these cases were lobectomy and 250 (19.7%) were pneumonectomy. The lobectomy / pneumonectomy rate was 4.1. The mean age of the pneumonectomy patients was 59 (range: 35 to 77) years. There were 32 patients (12.8%) aged under 50 years, 88 patients (35.2%) aged between 50 and 60 years, 98 patients (39.2%) aged between 60 and 70 years, and 32 patients (12.8%) aged above 70 years. The majority of the patients were males (94.8%), and the most common histopathological type was squamous-cell carcinomas (72.8%). In terms of T status, most of the patients were T2 with 168 patients (67.2%). In addition, 55 patients (22%) had comorbidities. A total of 72 patients (28.8%) were operated following neoadjuvant chemotherapy, while postoperative 50 patients (20%) were found to have N2 disease. Also, 106 patients (42.4%) received adjuvant chemotherapy (Table 1). Adjuvant therapy was given to 27 patients (37.5%) who received neoadjuvant therapy. Most of the patients (70%) had left pneumonectomy and R0 resection was implemented at a rate of 97.2%.Table 1: Demographics and the characteristics of the study population and the survival data.
The mean follow-up was 36.2 ± 28.6 (range: 0.1 to 104.9) months, and the median survival was 48.1 months (95% C. I. 30.6 – 65.6 months). The five-year overall survival rate was 56.6% (Figure 1).
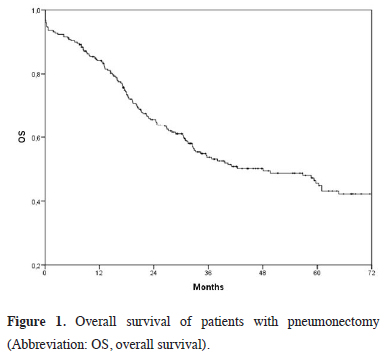 Click Here to Zoom |
Figure 1: Overall survival of patients with pneumonectomy (Abbreviation: OS, overall survival). |
The survival rates were lower in female patients (p = 0.06), non-squamous-cell carcinoma patients (p = 0.092), and those who did not receive adjuvant chemotherapy (p = 0.07); however, it did not show any statistical significance (Table 1). The difference in age groups (p = 0.002), detection of N2 disease (p = 0.001) and incomplete resection (p = 0.017) were found to significantly affect the survival rates. The five-year survival rate was 58.6% under 50 years of age, compared to 24.1% at the age of 70 and above (Figure 2).
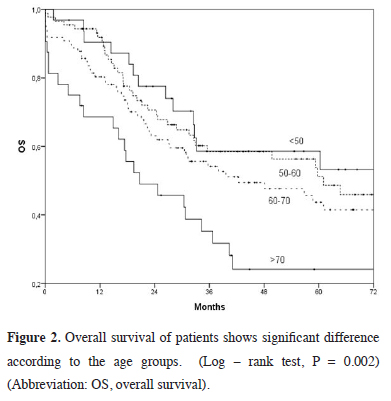 Click Here to Zoom |
Figure 2: Overall survival of patients shows significant difference according to the age groups. (Log – rank test, P = 0.002) (Abbreviation: OS, overall survival). |
The five-year survival rate was 28%, and median survival was 22.4 months for N2 disease (Figure 3) and 28.6% and 4.1 months, respectively for incomplete resection (Figure 4). The five-year survival was lower among patients receiving neoadjuvant therapy compared to those who did not (39.1% vs. 48.4%, p = 0.24).
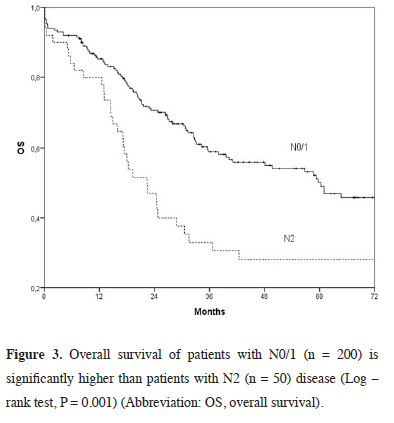 Click Here to Zoom |
Figure 3: Overall survival of patients with N0/1 (n = 200) is significantly higher than patients with N2 (n = 50) disease (Log – rank test, P = 0.001) (Abbreviation: OS, overall survival). |
 Click Here to Zoom |
Figure 4: Overall survival of patients with complete resection (n = 243) is significantly higher than patients with incomplete resection (n = 7) (Log – rank test, P = 0.02) (Abbreviation: OS, overall survival). |
During follow-up, disease progression was occurred in 80 patients (32%); 13 of them (16.2%) were local recurrence, 18 (22.5%) were both local recurrence and distant metastasis, and 49 (61.2%) were distant metastasis. Early rethoracotomy was performed in 14 patients (5.6%) within the postoperative 30 days; after left pneumonectomy in 10 and right pneumonectomy in four patients. The reasons for early rethoracotomy were bronchopleural fistula in seven patients, bleeding in six patients, and chylothorax in one patient. Six of the seven patients with fistula were cases with right pneumonectomy. Postoperative mortality occurred in four patients from early rethoracotomy group; two were due to bronchopleural fistula and two due to bleeding. The postoperative mortality rate after early rethoracotomy was 28.6% (4/14 patients).
The 30- and 90-day mortality rates were 6.4% and 7.6%, respectively. The review of national database revealed that 126 patients (50.4%) died and 70 of these patients (28%) died due to progression of NSCLC. Of the patients, 114 (45.6%) are alive and disease-free. The other 10 patients (4%) alive despite local recurrence or metastasis.
The Cox’s regression analysis revealed statistically significant results for the analyses related to age groups, nodal disease, complete resection, histology, and adjuvant therapy (Table 2).
The most influential factor in 30- and 90-day mortality was found to be age (p = 0.004 and p = 0.006, respectively) (Table 3), and the odds ratio from the logistic regression analysis was 3.3 (p = 0.001) and 2.5 (p = 0.003), respectively. The 30- and 90-day mortality in the presence of comorbidity was 12.7% and 14.5%, respectively (p = 0.05 and p = 0.04, respectively).
Table 3: Demographics and the characteristics of the study population and 30 and 90 days mortality.
Discussion
Pneumonectomy has a higher operative risk compared to more limited resections and there are different opinions about its effect on long-term survival [1]. Nevertheless, this procedure is still performed due to anatomic or technical reasons. Pneumonectomy accounts for 10 to 35% of all lung resections [2,3]. The tumors for which pneumonectomy is needed are often larger and centrally localized. Therefore, mediastinal lymph node metastasis are more common, and performing preoperative invasive staging is important for these patients.The former studies report that the five-year overall survival rate is 21 to 41% [1,2,4,5]. In this study, we found median survival to be 48.1 months and five-year overall survival rate to be 45.6% in 250 pneumonectomy cases. These rates were 48.8% and 44.3% for right and left pneumonectomy, respectively. The long-term overall survival rate for pneumonectomy was slightly higher in our study, than previously reported. This can be attributed to the fact that advanced stage diseases are detected more and surgical treatment is not performed on such patients in recent years as we have used advanced imaging techniques and invasive staging methods. This is also supported by our patient group 80% of which consisted of N0 and N1 patients. Similar two series reported higher postoperative N2 disease rate as 26% and 41% [1,6]. Of our patients with detected N2 disease, 60% had a squamous-cell carcinoma and 32% had an adenocarcinoma. This finding is contrary to some reports indicating that squamous cell carcinomas have a lower biological potential for mediastinal lymph node metastasis [6].
According to the tumor size in patients with postoperative N2 disease, it was found that 34% of the patients had a tumor <3 cm, whereas 66% had a tumor >3 cm in diameters. This finding suggests that there is a link between increased tumor size and N2 detection. The subgroup analyses of our study revealed that advanced age, incomplete resection and N2 disease have a significantly negative effect on long-term survival, whereas sex, histology, tumor size, the side of the operation, presence of comorbidity and adjuvant or neoadjuvant therapy did not have any statistically significant effect. The age group analysis showed that survival rates were higher in the younger group. The five-year survival rate was 58.6% among patients under 50 years of age compared to 24.1% among patients the age of 70 and above. It is known that R0 resection is one of the most important variables affecting survival. The rate of patients with detected R1 is reported to vary between 1.2% and 10.6% in various series [5,7]. This rate was 2.8% in our study and only one patient received neoadjuvant therapy. In our study, the five-year survival rate was 28.6% in R1 group compared to 46.1% in R0 group (p = 0.017). This can be considered as a proof that microresidual disease is an indicator of poor prognosis.
Currently, the lobectomy/pneumonectomy rate is on the increase due to sleeve lobectomies that are performed as an alternative to pneumonectomy. The lobectomy/pneumonectomy rate was four in our series. There are series with such rate of 4.9 in consistent with our study [5]; however, we believe that this rate should be further higher. A study that investigated the chronological changes in lung cancer surgery during the past 41 years found that the lobectomy/pneumonectomy rate has increased to 15.6 from 5 [4]. Similarly, the sublobar resections has increased (3% vs. 38%), whereas the pneumonectomy rate has significantly reduced (16% vs. 4%).
It is important for surgeons to reduce the higher morbidity and mortality risk of pneumonectomy and identify the causes of early postoperative deaths. Our study demonstrated that 19.7% of the resections performed due to NSCLC were pneumonectomy, and the 30- and 90-day overall mortality rates were 6.4% and 7.6%, respectively. These values are in strong agreement with the results of studies conducted at some experienced centers, which were published within the past five years (Table 4). However, early mortality rates are expected to be even lower upon developing technology and novel surgical methods and more modernized intensive care units. In the literature, an analysis including 1949 and 2002 reports showed that post-pneumonectomy 30-day overall mortality varied between 3.1 and 17% [8], whereas this rate has been reported to vary between 3.2% and 8.3% for the past five years [9-12].
Table 4: Mortality rates of pneumonectomy for nonsmall cell lung cancer in different series.
Furthermore, the present study also showed that advanced age had an adverse effect on mortality. The analysis of 30-day mortality revealed that there was no patient aged under 50, and 14 of 16 patients died were above the age of 60. Post-pneumonectomy 30-day mortality was significantly higher among elderly patients, compared to other resections [1,3]. Therefore, it is obvious that a more serious care is required for elderly patients. A study examining post-pneumonectomy prognosis among elderly lung cancer patients reported a significantly higher level of pneumonia and bronchial fistula in the older patient group compared to the younger patient group [13]. In addition, the aforementioned study showed a significantly higher rate of operation-related mortality in the older patient group.
Nonetheless, the role of neoadjuvant therapy is still questionable. Some series report lower postoperative mortality following neoadjuvant therapy [2], while others report higher early postoperative mortality [14] or higher mortality only in right pneumonectomy [15]. Our study showed a statistically significant difference in early mortality following neoadjuvant therapy. However, contrary to many other studies, we found 30-day mortality lower among patients that received neoadjuvant therapy compared to those who had pneumonectomy without any preoperative therapy (2.8% vs. 7.9%, p = 0.16). This difference is likely to result from the fact that the patients to whom surgery was recommended following neoadjuvant therapy were selected among patients with best conditional status. A meta-analysis carried out between 1990 and 2010 analyzed 27 studies and found the mean 30-day mortality rate for pneumonectomy following neoadjuvant therapy to be 7% [16]. The study emphasized higher 30- and 90-day mortality in right pneumonectomies compared to left pneumonectomies. Although there was no significant difference in the morbidity rates, some authors reported that early mortality was significantly higher in right pneumonectomies [9]. Similarly, we also observed higher early mortality rates in right pneumonectomies (9.3% vs. 5.1%, p = 0.26). The common reason for high mortality in right pneumonectomy include more bronchopleural fistula rate at the right side, the postoperative cough not being adequately effective due to the lengthiness and position of the left main bronchus, and the functional predominance of the ablated lung [2,11]. Among our study population, 10 of 14 patients undergoing early rethoracotomy following pneumonectomy had right pneumonectomy. Additionally, six of seven patients undergoing rethoracotomy due to bronchopleural fistula also had right pneumonectomy. This indicates that right pneumonectomy is associated with higher morbidity and mortality.
In conclusion, based on the findings from our single-center study, we suggest that preoperative evaluation should be made more meticulously and invasive staging should be performed to exclude N2 disease in all patients especially in advanced-age indicated for pneumonectomy. As early mortality following neoadjuvant therapy seems low, pneumonectomy can be performed safely in these patients. In addition, it should be kept in mind that pneumonectomy is likely to be curative when not avoidable, when performed by experienced professionals following a thorough assessment.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Reference
1) Riquet M, Mordant P, Pricopi C, Legras A, Foucault C, Dujon A et al. A rewiev of 250 ten year survivors after pneumonectomy for nonsmall cell lung cancer. Eur J Cardiothorac Surg 2014; 45: 876-81.
2) Melek H, Erol MM, Bayram AS, Çetinkaya G, Evrensel T, Sarıhan S et al. Pneumonectomy after neoadjuvant treatment. Turk Gogus Kalp Dama 2014; 22: 777-84.
3) Pei G, Zhou S, Han Y, Liu Z, Xu S. Risk factors for postoperative complications after lung resection for non-small cell lung cancer in elderly patients at a single institution in China. J Thorac Dis 2014; 6: 1230-8.
4) Nakamura H, Sakai H, Kimura H, Miyazawa T, Marushima H, Saji H. Chronological changes in lung cancer surgery in a single Japanese institution. Onco Targets and Therapy 2017; 10: 1459-64.
5) Thorsteinsson H, Alexandersson A, Oskarsdottir GN, Skuladottir R, Isaksson HJ, Jonsson S et al. Resection rate and outcome of pulmonary resections for non - small - cell lung cancer. J Thorac Oncol 2012; 7: 1164-9.
6) Mizushima Y, Noto H, Kusajima Y, Yamashita R, Sugiyama S, Kashii T et al. Results of pneumonectomy for non - small cell lung cancer. Acta Oncologica 1997; 36: 493-7.
7) Lequaglie C, Conti B, Massone PPB, Giudice G. Unsuspected residual disease at the resection magrin after surgery for lung cancer: fate of patients after long – term folow – up. Eur J Cardiothorac Surg 2003; 23: 229-32.
8) Watanabe S, Asamura H, Suzuki K, Tsuchiya R. Recent results of postoperative mortality for surgical resections in lung cancer. Ann Thorac Surg 2004; 78: 999-1003.
9) Frick AE, Lüders H, Leschber G. Thirty and 90-day mortality after lung cancer resection in 2242 patients. Annals of Oncology 2015; 26: 18-23.
10) Powell HA, Tata LJ, Baldwin DR, Stanley RA, Khakwani A, Hubbard RB. Early mortality after surgical resection for lung cancer: an analysis of the English National Lung cancer audit. Thorax 2013; 68: 826-34.
11) Pricopi C, Mordant P, Rivera C, Arame A, Foucault C, Dujon A et al. Postoperative morbidity and mortality after pneumonectomy: a 30-year experience of 2064 consecutive patients. Interact Cardiovasc Thorac Surg 2015; 20: 316-21.
12) Pezzi CM, Malin K, Mendez AS, Gay EG, Putnam JB. Ninety – day mortality after resection for lung cancer is nearly double 30 – day mortality. J Thorac Cardiovasc Surg 2014; 148: 2269-78.
13) Mizushima Y, Noto H, Sugiyama S, Kusajima Y, Yamashita R, Kashii T et al. Survival and prognosis after pneumonectomy for lung cancer in the elderly. Ann Thorac Surg 1997; 64: 193-8.
14) Bernard A, Deschamps C, Allen MS, Miller DL, Trastek VF, Jenkins GD et al. Pneumonectomy for malignant disease: factors affecting early morbidity and mortality. J Thorac Cardiovasc Surg 2001; 121: 1076-82.



