

2Department of Thoracic Surgery, Mardin State Hospital, Mardin, Turkey
3Department of Thoracic Surgery, Istinye Faculty of Medicine, Liv Hospital Ulus, Istanbul, Turkey DOI : 10.26663/cts.2019.00015
Summary
Background: Thymic masses are the most common anterior mediastinal neoplasms. In this study, we analyzed the histopathologic subgroups and stages of thymic tumors, evaluated mortality and morbidity, and identified the factors affecting survival in patients who underwent thymectomy due to thymic tumors.Materials and Methods: 159 patients who underwent surgery in our center for anterior mediastinal mass between January 2005 and December 2016 were analyzed retrospectively. Of the patients who had thymectomy procedures, 76 with thymoma or thymic carcinoma were included in the study.
Results: The mean age of the patients was 51 ± 14.3 (18-77) years. Forty-seven patients (61.8%) were male, and 29 (38.2%) were female. Fourteen of the patients (18.4%) were aged 65 years or older. Sixteen patients (21.1%) also had myasthenia gravis. The diagnosis was thymoma in 67 patients (85.2%) and thymic carcinoma in 9 patients (11.8%). There were no statistically significant differences in survival outcomes based on Masaoka or World Health Organization (WHO) thymoma stage. In terms of the extent of resection, survival time was 83.4 ± 10.5 months for patients who had simple thymectomy and 124 ± 5.4 months among patients who underwent extended or maximal thymectomy (p = 0.043).
Conclusions: Our findings suggest that thymoma stage and histopathologic subgroup were not prognostic factors, while extended resection was the most significant factor in survival.
Introduction
Thymic tumors are rare tumors originating from thymic epithelial cells and are the most common neoplasm of the anterior mediastinal compartment. The incidence of thymoma was reported as 0.13 per 100,000 person-years in the United States [1]. Thymic tumors may be asymptomatic and detected incidentally or can cause symptoms related to mass effect or invasion of adjacent structures upon reaching a sufficient size. They may also present with symptoms associated with concomitant myasthenia gravis or other paraneoplastic conditions. Approximately 40% of patients with thymoma develop systemic paraneoplastic conditions [2].Complete surgical resection is recommended for all thymic tumors due to their malignant potential. Thymic cells can also be detected microscopically in tissues adjacent to the thymus. The primary treatment for thymoma is surgical resection. Complete resection is a major prognostic factor affecting survival [3]. There is no clear consensus on surgical technique. Methods such as sternotomy, video-assisted thoracoscopic surgery (VATS), thoracotomy, and robotic surgery are used. Although there have been recent publications reporting the results of minimally invasive procedures in early-stage thymomas, the long-term outcomes have not been determined [2,3].
In the present study, we evaluated early and long-term morbidity and prognostic factors associated with survival in patients who underwent surgery for thymoma.
Methods
The records of 159 patients who underwent surgery for an anterior mediastinal mass in Yedikule Chest Diseases Hospital between January 2005 and December 2016 were analyzed retrospectively. A total of 76 patients were included in the study. The study was approved by the institutional review board and was conducted in accordance with the principles of the Declaration of Helsinki (version no: 1118, 09/11/2017). The flowchart of patient inclusion and exclusion is shown in Figure 1.
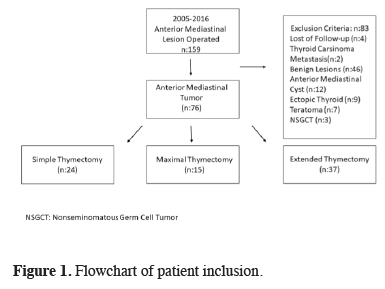 Click Here to Zoom |
Figure 1: Flowchart of patient inclusion. |
Preoperative Evaluation
All patients were evaluated for pulmonary function and cardiac pathology before surgery. Radiological evaluation was performed with chest x-ray and thoracic computed tomography (CT), and magnetic resonance imaging (MRI) and positron emission tomography–CT (PET/CT) was added to the preoperative examination when deemed necessary. All patients underwent routine preoperative biochemical analysis and electrocardiogram (ECG); echocardiogram (ECHO) was ordered when needed. All patients with suspected anterior mediastinal mass were evaluated for myasthenia gravis by a neurologist. Patients who were newly diagnosed with myasthenia gravis received medical therapy and underwent surgery after stabilization.
Comorbidities were categorized in four groups as respiratory (chronic obstructive pulmonary disease, asthma), cardiac (past myocardial infarction, arrhythmia, and history of the stent); endocrine (diabetes mellitus, goiter), and neurologic (myasthenia gravis).
Patients with no distant metastasis in the clinical and radiological evaluation and whose tumors were considered resectable underwent surgical resection. VATS, thoracotomy, or sternotomy was performed based on the radiologic location and size of the tumor and suspicion of invasion of adjacent tissues.
Surgical Approach
Simple thymectomy was defined as complete removal of only the mass and thymus by VATS, thoracotomy, or sternotomy.
Extended thymectomy was defined as resection of the thymus together with the surrounding pericardial/diaphragmatic adipose tissues to the bilateral phrenic nerve in the horizontal axis and the area from the thyroid to the diaphragm in the axial axis by VATS, thoracotomy, or sternotomy (Figure 2).
 Click Here to Zoom |
Figure 2: Material resected in extended thymectomy. |
Maximal thymectomy was defined as extended thymectomy via sternotomy with the additional dissection of the cervical, paratracheal, subcarinal, and aorticopulmonary lymph nodes.
Any detected invasion of the pericardium, parenchyma, pleura, or vasculature was included completely within the surgical margins.
Postoperative Follow-up
Based on the histopathological diagnosis, the patients were separated into two main groups, thymoma, and thymic carcinoma. Thymoma staging was done according to the World Health Organization (WHO) [4] and Masaoka [5] staging systems. Of the recorded complications, we evaluated morbidities that occurred in-hospital or within the first postoperative month. Atelectasis, hemorrhage, pneumonia, atrial fibrillation managed with medical treatment, surgical site infections requiring revision, and hoarseness were accepted as morbidities.
The groups were further classified by histopathologic subgroup and stage. Mortality, morbidity, and factors believed to be associated with survival were analyzed.
Statistical Analysis
The SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) software package was used for statistical analyses. As descriptive statistics, the Chi-square (χ2) test was used to calculate frequency values. A t-test was used to compare means of independent groups and the Mann-Whitney U test was used to compare medians. Survival analysis was conducted using the Kaplan-Meier method and curves were compared using the log-rank test. Prognostic factors were analyzed with multivariate analysis using Cox’s proportional hazard model to adjust for potential confounding factors. The statistical significance level was accepted as p = 0.05 for all analyses.
Results
Of the 76 patients included in the study, 47 (61.8%) were male, and 29 (38.2%) were female. The mean age was 50 ± 13.8 (18-81) years. Fourteen of the patients (18.4%) were aged 65 years or older. Sixteen patients (21.1%) were also diagnosed with myasthenia gravis. There were 45 smokers in the patient group (59.2%). Thirty-four (44.7%) of the 76 patients included in the study had comorbid diseases, the most common of which were myasthenia gravis and cardiac comorbidities. The patients’ demographic data are summarized in Table 1.Table 1. Demographic characteristic of patients with thymic tumors.
Six patients (7.9%) with a suspected invasion of major vascular structures were given neoadjuvant radiotherapy. All 6 of these patients underwent sternotomy after neoadjuvant therapy, and complete resection was achieved. Fifty-eight patients (76.3%) underwent sternotomy, 8 (10.5%) underwent VATS, and 10 (13.2%) underwent thoracotomy. Comparison of tumor size according to surgical approach showed that mean tumor diameter was 28.8 ± 3.9 (10-48) mm in patients selected for VATS, 69.2 ± 15.9 (0.1-150) mm in those selected for sternotomy, and 66.8 ± 4.1 (10-175) mm among those selected for thoracotomy (p = 0.003).
Twenty-four (31.6%) of the patients underwent simple thymectomy, 37 (48.7%) underwent extended thymectomy, and the remaining 15 patients (19.7%) underwent maximal thymectomy. In addition to thymectomy, lung parenchymal wedge resection was performed in 7 patients, left upper lobectomy in 1 patient, left innominate vein graft in 1 patient, and superior vena cava resection in 1 patient. The mean length of hospital stay was 6.4 ± 6.4 (2-45) days. The surgical approach was not associated with length of hospital stay (p = 0.057).
Postoperatively, 33 complications occurred in 25 (32.9%) of the 76 patients. No significant difference in complication rates was observed according to age, sex, surgical approach (VATS, thoracotomy, sternotomy) or extent of resection (simple, extended, maximal) (p > 0.05). Atelectasis occurred in nine patients, hemorrhage requiring revision in 1 patient, pneumonia in 4 patients, medically managed atrial fibrillation in 7 patients, deep surgical site infection in 7 patients, persistent hoarseness in 1 patient, and diaphragmatic eventration due to phrenic nerve injury in 4 patients.
A statistically significant difference in complication rate was detected between patients with and without comorbidities (17 patients vs. eight patients, respectively; p = 0.05). When the relationship between myasthenia gravis and postoperative complications was examined, it was seen that 8 (50%) of 16 patients with myasthenia gravis developed complications (p = 0.099). Of the 14 patients with endocrine comorbidities (diabetes, hypothyroidism), eight (57.1%) developed postoperative complications (p = 0.04). Length of hospital stay was significantly longer in patients who developed complications (p < 0.001) (Table 2).
Table 2. Evaluation of factors affecting the morbidity.
When divided based on histopathology, 67 (85.2%) of the patients had a thymoma, and 9 (11.8%) had thymic carcinoma. All subgroups based on thymic tumor stage are shown in Table 3.
Table 3. Masaoka staging and WHO classification of pa-tients with thymoma.
There was no intraoperative mortality in this series. The 30-day mortality rate was 1.3% (n = 1) and the 3-month mortality rate was 2.6% (n = 2). All three of the deceased patients underwent sternotomy; one was lost in the first postoperative month due to a myasthenic crisis. Another patient died due to respiratory failure after pneumonia, and the other was diagnosed with myasthenia gravis and died due to other problems.
11 (45.8%) of the patients who underwent simple thymectomy according to Masaoka stages were stage 1, 10 (41.7%) were stage 2, and 3 (12.5%) were stage 3. Masoka stages of the patients who underwent extended or maximal thymectomy were 23 patients (44.2%) stage 1, 22 patients (42.3%) were stage 2, seven patients (13.2%) were stage 3. There was no statistically significant difference between these groups (p = 0.989).
The mean follow-up time was 62.6 ± 34.1 months. Mean overall survival was 118 ± 5.5 months. The 5-year survival rate was 83.3%, and 10-year survival was 79.4%. Advanced age and extent of resection were identified as prognostic factors affecting survival (Table 4) (Figure 3).
Table 4. Prognostic factors affecting the survival of patients with thymic tumors.
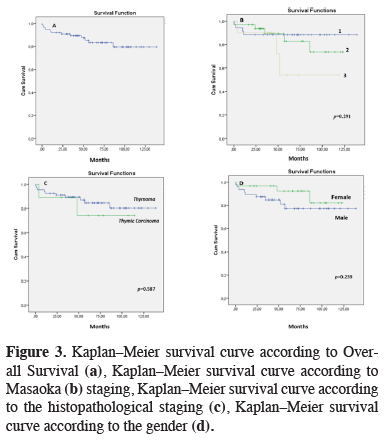 Click Here to Zoom |
Figure 3: Kaplan–Meier survival curve according to Overall Survival (a), Kaplan–Meier survival curve according to Masaoka (b) staging, Kaplan–Meier survival curve according to the histopathological staging (c), Kaplan–Meier survival curve according to the gender (d). |
Recurrence occurred in three patients (3.9%), of whom 2 had thymic carcinoma, and 1 had type B3 thymoma. There was a significant association between histopathologic stage and recurrence (p = 0.03). Survival analysis, according to the extent of resection, showed that mean survival time was 83.4 ± 10.5 months in the simple thymectomy group and 124 ± 5.4 months in the other groups (extended and maximal thymectomy) (p = 0.043).
Discussion
Determining the actual incidence of anterior mediastinal masses from the medical literature can be difficult for several reasons. One of these is the varying clinical and radiological classifications used to define the mediastinal compartments. Another reason is that non-neoplastic lesions such as thymic cyst and pericardial cyst are included in these numbers. Despite the difficulty of determining their exact incidence, approximately 35% of anterior mediastinal lesions are thymic malignancies [6-8]. Among the total patient population screened for inclusion in this study, the incidence of thymic epithelial malignancy was 76 in 159 patients (47.7%).Thymoma is reported to affect both sexes equally and usually appears in the fourth or fifth decades [9]. In our patient group, 61.9% were men, and the mean age was 50 ± 13.8 (18-81) years, consistent with the literature.
Surgery is the cornerstone of thymoma treatment, being the only curative method. The goal is to achieve complete surgical resection, which requires resection of possible tumor spread outside the thymus. Partial or full sternotomy for complete thymectomy is the main surgical approach in thymus surgery. For advanced tumors, additional incisions should be used when necessary to achieve complete resection, especially in the presence of lung or pleural space invasion. Some investigators reported no local recurrence in patients without microscopic invasion of the capsule and surrounding tissues, while others have reported local recurrence at rates of 2–10%. Thymomas can be invasive and metastatic. Therefore, surgical procedures should be planned, taking into account the malignant potential of all thymomas [10]. In our study, the rate of local recurrence was 3.9%. Two of the local recurrences were thymic carcinoma, and one was type B3 thymoma.
Procedures and standards of minimally invasive methods were defined by the International Thymic Malignancy Interest Group (ITMIG) in 2011 to facilitate systematic data collection [11]. Minimally invasive thymus resections were already being performed in our center and were adapted to conform to this new framework of standards following the ITMIG workshops. In our clinic, we perform extended thymectomy by VATS in patients with tumors that are 3 cm in size, stage 1-2, and assessed as resectable using VATS. The feasibility of extended thymectomy via VATS has been debated, and the concept of videothoracoscopic extended thymectomy has been described. We believe that inexperienced centers, VATS thymectomy is appropriate for thymomas up to 3 cm in size provided that complete resection is achieved.
The coexistence of specific autoimmune diseases with thymoma, especially myasthenia gravis, must be evaluated before surgery. The incidence of thymoma in patients with myasthenia gravis is known to be 10–15%, while approximately 30% of patients with thymoma are reported to have myasthenia gravis [12]. Some publications report that myasthenia gravis in patients with thymoma is associated with a poorer prognosis, while others have reported that prognosis is not adversely affected. In a multicenter study of 1089 patients, Kondo and Monden reported that thymomas associated with myasthenia gravis were diagnosed earlier and had a lower rate of recurrence due to their less aggressive nature; therefore, they claimed that the presence of myasthenia gravis could be considered a favorable prognostic factor for thymoma [13-15]. Of all the thymoma patients in our series, 16 (21.1%) were diagnosed with myasthenia gravis. The 5- and 10-year survival rates of patients with myasthenia gravis were 81.3% and 60.9% (p = 0.263).
The most significant prognostic factor in thymoma is complete resection. Another prognostic factor accepted by many researchers is the quality of the surgery [15]. In a study by Cowen et al. [16], the metastatic spread was significantly more common among patients who did not undergo complete surgical treatment (had an only biopsy or subtotal resection) when compared with patients who underwent complete resection. A complete resection rate of 100% was reported in patients with stage I thymoma, and this success rate was found to decrease as stage increased [17]. According to Detterbeck et al. [18], complete resection results in significant improvement in survival for patients with stage III tumors in particular. In the present study, we observed no significant difference in survival based on Masaoka staging (p = 0.291). However, there was a trend toward poorer survival outcomes as the stage increased. We attribute the discrepancy between our findings and those reported in the literature to our small patient number. Advanced age and extent of resection were identified as factors associated with survival in our study.
In addition to thymoma staging, histopathology can also be useful in determining tumor resectability. Nearly all WHO type A thymomas are noninvasive tumors and should be treated with complete resection. Type B thymomas are often invasive, and the timing of the presentation is very important. Effort should be made to resect as completely as possible. Although complete resection is achieved in 92% of these tumors, recurrence has been reported at a rate of 29% [19]. In the present study, recurrence was observed in a total of 3 patients (3.9%), 2 with thymic carcinoma, and 1 with type B3 thymoma (p = 0.03). All three of these patients had stage III disease. We detected a close association between histopathological stage and the likelihood of recurrence. As with Masaoka stage, there was no significant difference in survival between the histological subgroups of thymomas compared to thymic carcinomas according to WHO stage (p = 0.587).
There were no statistically significant differences in survival outcomes based on Masaoka or WHO thymoma stage. In terms of the extent of resection, survival time was 83.4 ± 10.5 months in the simple thymectomy group versus 124 ± 5.4 months in the combined extended and maximal thymectomy group (p = 0.043). Based on this finding, we believe that regardless of stage or histopathologic subtype, complete resection of thymomas with at least extended resection is a key factor in survival.
The main limitations of our study were that it included a small number of patients, multiple surgeons performed the procedures, and the general status of the patients was not assessed preoperatively. In addition, we were not able to demonstrate a statistically significant effect of thymic carcinoma histopathologic subgroup and survival. A large number of subgroups resulted in insufficient patient numbers in each group.
As a conclusion, although not significant, the tumor stage was found to be a poor prognostic factor, while complete resection and advanced age were the most significant prognostic factors identified in our study. All of the patients in our study underwent complete resection, and survival outcomes were acceptable, independent of stage and histopathological features. However, patients who underwent simple thymectomy had significantly lower survival rates despite a complete resection. This suggests that regardless of stage or histopathologic subtype, complete resection of thymomas with at least extended resection is a key factor in survival.
Declaration of conflicting interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support.
Reference
1) Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010; 5: S260-5.
2) Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J (Engl) 2013; 126: 2186-91.
3) Sunil S, Kaiser LR. Surgery For Myasthenia Gravis. In: Watson TJ, Peters JH, Patterson GA, editors. Pearson’s Thorac. Esophageal Surg. 39th ed., Elsevier; 2008, p. 1549-61.
4) Girard N, Harris NL, Jaffe ES. The 2015 WHO Classification of Tumors of the Thymus: Continuity and Changes 2016; 10: 1383-95.
5) Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981; 48: 2485-92.
6) Davis Jr RD, Oldham Jr HN, Sabiston Jr DC. Primary cysts and neoplasms of the mediastinum: recent changes in clinical presentation, methods of diagnosis, management, and results. Ann Thorac Surg 1987; 44: 229-37.
7) Rubush JL, Gardner IR, Boyd WC, Ehrenhaft JL. Mediastinal tumors. Review of 186 cases. J Thorac Cardiovasc Surg 1973; 65: 216.
8) Mullen B, Richardson JD. Primary anterior mediastinal tumors in children and adults. Ann Thorac Surg 1986; 42: 338-45.
9) Blumberg D, Port JL, Weksler B, Delgado R, Rosai J, Bains MS, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg 1995; 60: 908-14.
10) Cohen DJ, Ronnigen LD, Graeber GM, Deshong JL, Jaffin J, Burge JR, et al. Management of patients with malignant thymoma. J Thorac Cardiovasc Surg 1984; 87: 301-7.
11) Toker A, Sonett J, Zielinski M, Rea F, Tomulescu V, Detterbeck FC. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011; 6: S1739-42.
12) Drachman DB. Myasthenia gravis. N Engl J Med 1978; 298: 136-42.
13) Kondo K, Monden Y. Thymoma and myasthenia gravis: a clinical study of 1,089 patients from Japan. Ann Thorac Surg 2005; 79:2 19-24.
14) Evoli A, Minisci C, Di Schino C, Marsili F, Punzi C, Batocchi AP, et al. Thymoma in patients with MG characteristics and long-term outcome. Neurology 2002; 59: 1844-50.
15) Tilkan OK, Karakurt Ö, Demircan S. Timus Patolojilerinin Cerrahisinde Klinik Deneyimimiz. Zonguldak Karaelmas Üniversitesi Tıp Fakültesi Derg. Medi ForuM 2014: 1: 1-18.
16) Cowen D, Richaud P, Mornex F, Bachelot T, Jung GM, Mirabel X, et al. Thymoma: results of a multicentric retrospective series of 149 non-metastatic irradiated patients and review of the literature. Radiother Oncol 1995;34: 9-16.
17) Detterbeck FC, Parsons AM. Thymic tumors. Ann Thorac Surg 2004; 77: 1860-9.



